A drug delivery system (DDS) is any formulation, device or technology that carries a therapeutic agent from its source to a patient and controls the rate, timing, and site of drug release to achieve a desired therapeutic outcome. Modern DDSs range from traditional oral tablets and injectables to engineered controlled-release implants, inhalers, transdermal patches, and advanced nanoparticle-based targeted carriers. Drug delivery is now an intersection of pharmaceutics, materials science, engineering, regulatory strategy and digital health — and this convergence is reshaping drug efficacy, safety, patient adherence and market potential. [4,7]
This long-form guide explains what drug delivery systems are, their types and technologies, regulatory and market considerations, real-world examples, step-by-step development/validation checkpoints, cost/ROI signals for manufacturers and distributors, and practical next steps you can act on. Wherever possible I cite authoritative sources (regulators, review articles, market intelligence) so you can verify and reuse content.

Table of Contents
Definition & why drug delivery systems matter
Definition (concise): A drug delivery system is the combination of formulation and/or device that enables a medicine to reach its target with controlled kinetics and acceptable safety, tolerability and stability. The aim is to increase therapeutic index (efficacy/safety), improve adherence, enable new routes (e.g., transdermal, inhalation), or protect labile drugs (e.g., proteins).[4]
Why it matters (business & clinical):
- Improves patient adherence (e.g., fewer doses via controlled release).[7]
- Enables biologics delivery that oral pills cannot (injectables, inhalers, patches).[4]
- Differentiates products for lifecycle management and market exclusivity (combination products & device patents).[2,10]
- Frequently increases product value — device-enabled drugs or delivery innovations are associated with premium pricing and faster adoption in specialty markets.[3,8]
Core principles & performance parameters
When designing or evaluating a DDS, these parameters guide decisions:
- Route of administration: oral, parenteral (IV/SC/IM), transdermal, pulmonary, nasal, ocular, buccal, vaginal, implantable, etc. Route shapes bioavailability and safety profile.[4]
- Release kinetics: immediate, sustained, controlled, pulsatile, or targeted/triggered. Each profile suits different therapeutic goals.[1]
- Site specificity / targeting: passive (e.g., EPR for tumors) or active (ligand-mediated targeting) to concentrate drug at action site.[1]
- Stability & protection: excipient selection or encapsulation to stabilize APIs (especially biologics).[4]
- Dose accuracy & device ergonomics: for devices, human factors and dose-accuracy matter for safety and regulatory acceptance. [10]
- Manufacturability & scale: cost, reproducibility, packaging, sterilization, and supply chain readiness.[6]
- Regulatory classification: device, drug, or combination product — determines dossier and review pathway.[2,10]
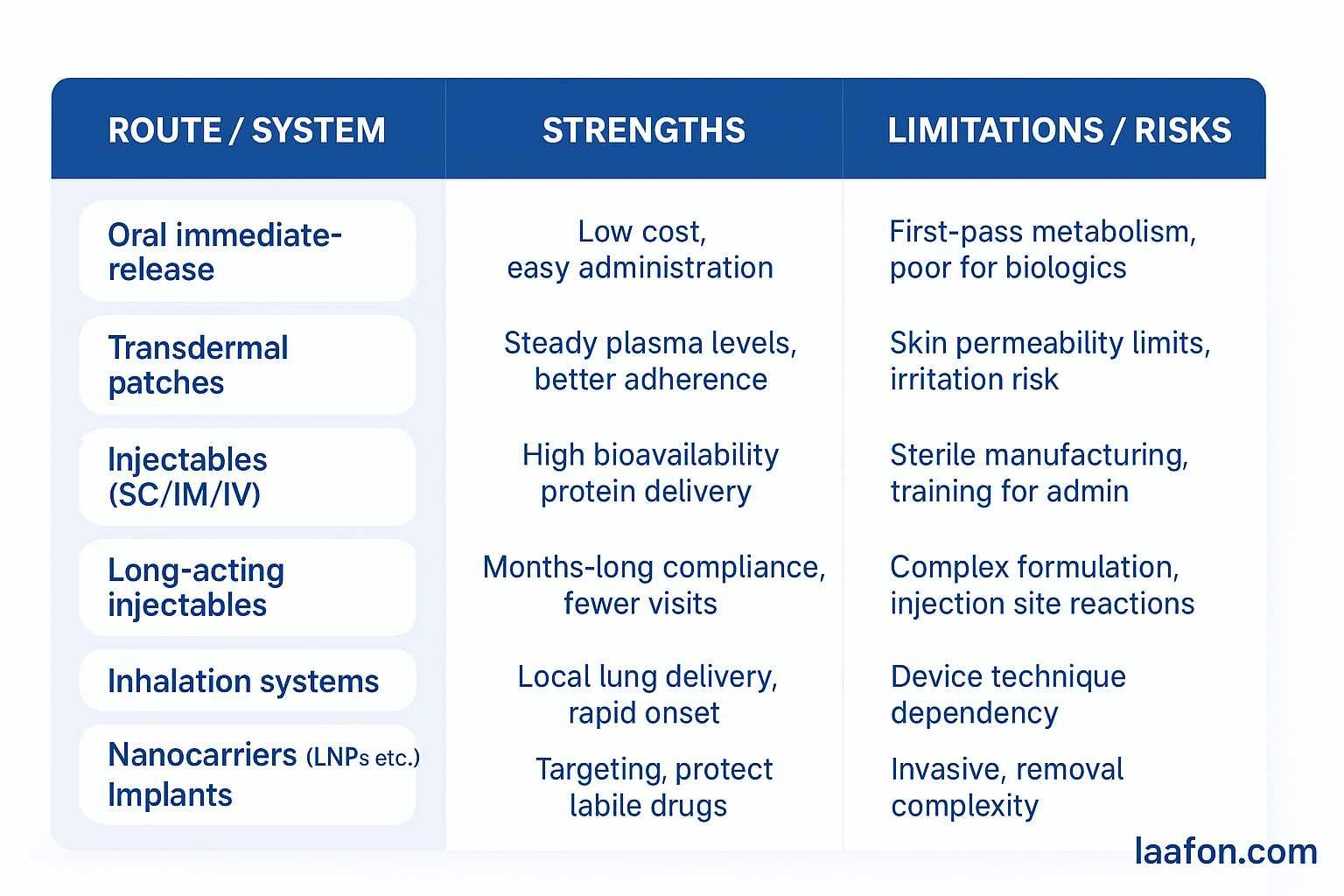
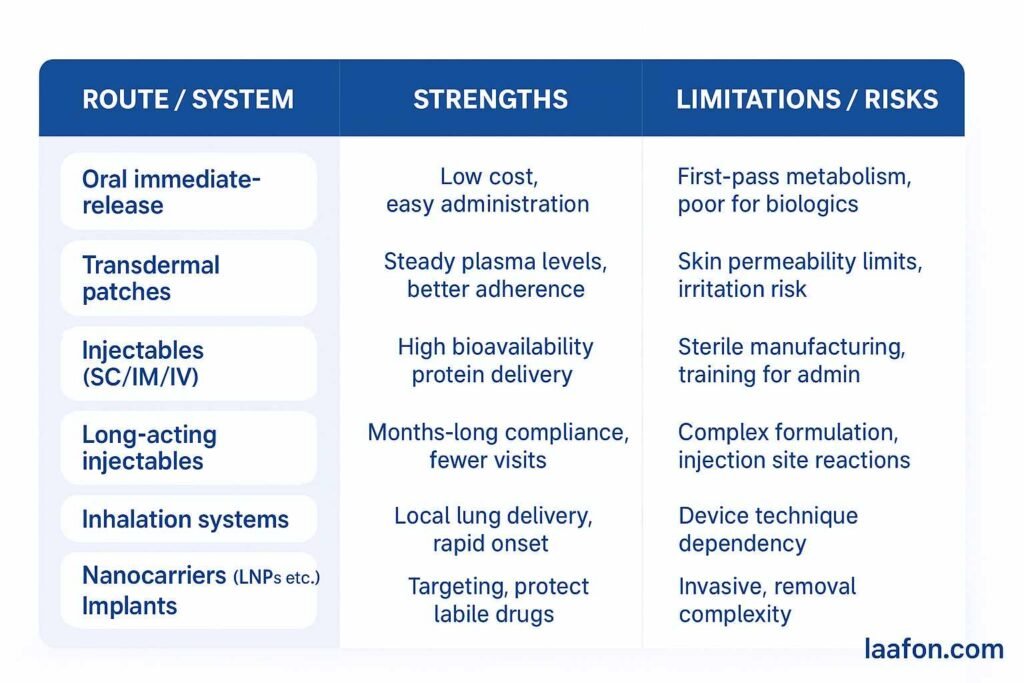
Types of drug delivery systems (taxonomy + short examples)
Below is a practical taxonomy (keywords embedded naturally):
A. Traditional/Conventional systems
- Oral immediate-release tablets and capsules — the baseline for systemic therapies.[4]
- Parenteral injections (IV/IM/SC) — sterile solutions or suspensions for rapid or controlled absorption.[4]
B. Controlled-release & sustained-release systems
- Matrix/tablet controlled release (hydrophilic matrices, coated pellets).
- Osmotic pumps (e.g., OROS) — consistent zero-order release for certain drugs.
C. Transdermal drug delivery systems (TDDS)
- Patches (reservoir, matrix) — e.g., fentanyl, nicotine, hormonal patches. Advantage: steady plasma levels, improved adherence.[7]
D. Inhalation & pulmonary delivery
- MDIs, DPIs, nebulizers — essential for respiratory drugs and increasingly for systemic biologic delivery via lungs.[4]
E. Implantable systems & injectable depots
- Biodegradable polymer implants (e.g., leuprolide implants), non-biodegradable reservoirs, long-acting injectable microspheres for sustained months-long release.
F. Targeted / nanocarrier systems
- Liposomes, lipid nanoparticles (LNPs), polymeric nanoparticles, dendrimers — enable targeted delivery, improved solubility and protection of nucleic acids / biologics. (LNPs are central to mRNA vaccines.)[1]
G. Combination products & delivery devices
- Pre-filled syringes, autoinjectors, inhalers, infusion pumps, wearable patches — device component critical for dosing and user safety; may be regulated as combination products.[2,6]
H. Emerging / advanced systems
- Microneedle arrays (minimally invasive transdermal), smart drug-delivery patches (with sensors and actuators), stimuli-responsive (pH, enzyme, temperature) releases, bioadhesive mucosal systems and cellular/viral vectors for targeted delivery.[4,7]
ALSO READ: Find the latest notifications about DPCO prices and CDSCo orders.
Key technologies and drug delivery methods (how they work)
This section matches the keyword drug delivery methods and drug delivery systems technology — short technical explanations.
1 Oral controlled-release (matrix / coating)
- Matrix systems: drug embedded in polymer; release by diffusion and matrix erosion.
- Coated multiparticulates: pellets with rate-controlling membranes; advantage is reduced variability and reproducible scale-up.
2 Transdermal (patch) technology
- Mechanism: drug diffuses through stratum corneum; enhancers, micro-reservoirs and microneedles increase permeation and allow delivery of molecules not normally skin-permeable.[7]
3 Inhalation (aerosol physics)
- Mechanism: particle size (MMAD) determines where in the respiratory tract the particle deposits — key for local vs systemic effects. Device design (DPI vs MDI) controls dose reproducibility.
4 Long-acting injectables & implants
- Mechanism: biodegradable polymers (e.g., PLGA) encapsulate API; hydrolysis controls release over weeks to months.
5 Nanoparticle & lipid systems
- Mechanism: drug is encapsulated or chemically attached; surface ligands/PEGylation modify circulation time, cell uptake, and immune recognition. LNPs use ionizable lipids to encapsulate mRNA and assist endosomal escape.[1]
6 Smart / digital drug delivery devices
- Examples: pumps that adjust rates; Bluetooth-connected inhalers that log adherence; closed-loop insulin pumps. These combine hardware, firmware, and human factors design and often trigger combination-product regulatory review.[2,6]
Development & validation — step-by-step process (practical checklist)
This checklist is suitable for product teams (R&D, QA/RA, manufacturing):
Concept → Clinical product: 12 practical steps
- Target product profile (TPP): define route, dose, release profile, user population, stability targets.
- Preformulation & feasibility: API properties (solubility, pKa, stability), excipient screening, early prototypes.
- Prototype & engineering: device concept (if any), materials selection, human factors early testing.[10]
- In vitro performance testing: dissolution/release, aerosol characterization (for inhalers), permeation studies (for TDDS).[6]
- Sterilization/bioburden control plan (if needed): validate terminal sterilization or sterile assembly.
- Scale-up & process validation: equipment selection, process parameters, critical quality attributes (CQAs).
- Non-clinical (PK/PD and safety): animal models for exposure and local tolerance.
- Human factors/usability testing: critical for device safety and often required by regulators for combination products.[7]
- Clinical studies / Bioequivalence: depending on novelty — BE studies or clinical efficacy trials; special considerations for generics or alternative dosage forms.
- Regulatory submission: dossier aligning drugs and device sections (for combinations, follow FDA/EMA combination product guidelines).
- Post-market surveillance & vigilance: device malfunctions, adverse events, and product life-cycle updates.
- Product lifecycle management: improvements, digital add-ons, or new indications for competitive advantage.
Design control & documentation: For devices and combination products, FDA expects design inputs/outputs, risk management, verification/validation records — see FDA guidance “Essential Drug Delivery Outputs”.[6]
Regulatory landscape & documentation essentials
Key points for regulatory planning
- Classification: some DDS are drugs, others devices; many are combination products (drug + device) with shared review responsibilities — check FDA Office of Combination Products and EMA guidelines early.[2, 10]
- FDA expectations: performance data for drug delivery outputs, verification and validation, human factors, and device-specific risk analyses are increasingly enforced. See FDA guidance (Essential Drug Delivery Outputs, Combination Products guidance).[6]
- EMA: provides guidance on quality documentation and Q&A for combination products; Notified Body opinions and MDR must be considered for device components sold in EU.[10]
- WHO / Global procurement: for products targeting public health programs, alignment with WHO essential medicines and prequalification requirements is important for procurement and developing-country markets.[11]
Practical RA tip: classify as a combination product if the device and drug are marketed together, or if one is integral to the other’s function; early engagement with regulators is recommended to define the lead center (drug vs device) and clinical expectations.[2]
Market research: size, growth drivers, & ROI signals
Market size & growth (select figures):
- Several market reports put the global drug delivery systems market in the USD 45–50 billion range for 2024–2025, with projected CAGR in the 4–8% band depending on report and segment. (Examples: Fortune Business Insights valuation ≈ USD 46.23B in 2024; FactMR and ResearchAndMarkets show similar mid-decade expansions).[3,9 ]
- The drug delivery devices market (broader, includes pumps, inhalers, autoinjectors) is much larger — some reports estimate hundreds of billions (e.g., Grand View Research estimates ~USD 432B for 2024 across devices). That gap shows that device-enabled delivery and digital health integration are high-value areas.[8]
Growth drivers
- Rise of biologics and gene therapies requiring specialized delivery (injectables, LNPs).[1]
- Ageing populations and chronic therapies (long-acting injectables, implants).[9]
- Digital health / adherence tools and home-care trends (wearables, smart inhalers).[8]
ROI signals for manufacturers / distributors
- High ROI opportunities: device upgrades for existing drugs (autoinjectors for biologics), long-acting formulations that command premium prices, and combination products with usage data that enable payers to accept higher reimbursement.[3,8]
- Cost drivers / risks: development complexity (device + drug), need for human factors studies, higher regulatory burden and manufacturing complexities, sterilization and aseptic production for parenterals.[6]
Real-world examples & short case studies
- mRNA vaccines and LNPs: LNP technology enabled systemic delivery of nucleic acids (mRNA COVID-19 vaccines). This is a benchmark for how delivery tech can validate new therapeutic modalities.[1]
- Transdermal patches: nicotine and fentanyl patches demonstrate improved adherence and steady plasma levels. TDDS has a strong use case for chronic therapy and pain management.[7]
- Autoinjectors for biologics: many monoclonal antibodies have been reformulated into autoinjectors or prefilled syringes to enable self-administration and home use — a commercial win (reduced clinic costs, improved adherence). Regulatory scrutiny on human factors and device verification is high.
- Smart inhalers & adherence tracking: Bluetooth-enabled inhaler add-ons capture usage data and are being adopted in COPD/asthma disease-management programs. These are competitive differentiators for pharma brands.[8]
Comparison table — practical pros & cons
FAQs
What is the difference between a drug delivery system and a drug delivery device?
A drug delivery system refers to the overall formulation + method (e.g., an implant that releases drug over six months). A drug delivery device is the hardware component (e.g., autoinjector, inhaler, pump) used to administer or control that delivery. Combination products combine both and often have unique regulatory pathways. [4]
Are nanoparticle carriers safe for clinical use?
Many nanoparticle platforms (liposomes, some polymeric NPs, LNPs) are clinically used, but safety depends on materials, biodistribution and immunogenicity; robust preclinical toxicology and clinical monitoring are required. LNPs for mRNA vaccines are a modern example of successful clinical translation.[1]
How do regulators assess combination products?
Regulators determine the primary mode of action (drug vs device) to assign lead center and expect integrated evidence covering chemistry/manufacturing (CMC), device performance, human factors, and clinical safety/effectiveness. Early regulator engagement is advised.
What are major cost drivers for delivering a new DDS?
R&D (formulation and device engineering), sterile or controlled manufacturing, device tooling and validation, clinical studies required, human factors testing, and regulatory submissions. These can be offset by premium pricing for unique delivery features and extended commercial exclusivity.
Conclusion
Drug delivery systems are a strategic capability — they transform APIs into usable, safer, and more commercially valuable products. Whether you are a manufacturer, distributor, or product manager, investing in the right delivery approach can increase adherence, enable new indications, reduce healthcare costs and command premium pricing.
Action steps (practical):
- Map your API to delivery needs: run a 1-page TPP within 1 week to shortlist candidate routes.
- Regulatory triage: classify as device/drug/combination and request a pre-submission meeting with the regulator (FDA/EMA) early. [2,6]
- Feasibility sprint: perform accelerated preformulation (solubility, stability, permeability) and 2-3 prototype concepts.
- Human factors plan: for any device, schedule human-factor/usability testing early.[2]
- Market check: run a short ROI model: incremental price premium × expected uptake vs development cost — focus on autoinjectors, long-acting injectables, and smart devices for highest short-term ROI.[8]
References:
- Tiwari G, et al. Drug delivery systems: An updated review. International Journal of Pharmaceutical Investigation. (review). PMC article. PMC
- FDA — Combination Products guidance & Office of Combination Products. U.S. Food & Drug Administration. U.S. Food and Drug Administration
- Fortune Business Insights — Drug Delivery Systems Market Size (2024 report). Market data and forecasts. Fortune Business Insights
- ScienceDirect / Drug Delivery System overview (topic page on ScienceDirect). ScienceDirect
- Crasta A., 2025. Review: Transdermal drug delivery system (2025 review). ScienceDirect. ScienceDirect
- FDA guidance — Essential Drug Delivery Outputs for Devices Intended to Deliver Drugs and Biological Products. (2024 PDF guidance). U.S. Food and Drug Administration
- Biomaterials Research — Recent advances in transdermal drug delivery systems (2021). BioMed Central
- Grand View Research — Drug Delivery Devices Market Size (2024 report). Grand View Research
- FactMR / Research reports — Drug Delivery Systems Market Forecasts. Fact.MR
- EMA — Guideline on quality requirements for drug-device combinations; Q&A documents and updates (2019–2024). European Medicines Agency (EMA)
- WHO — Essential Medicines / Model List resources. World Health Organization