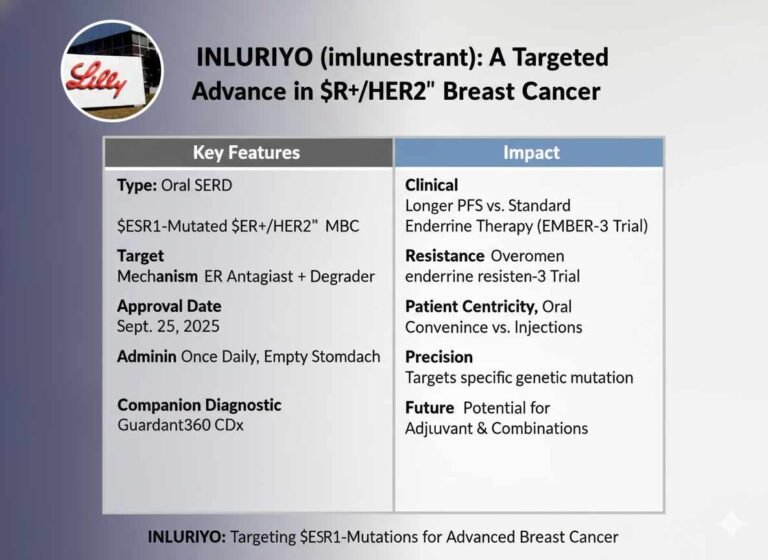
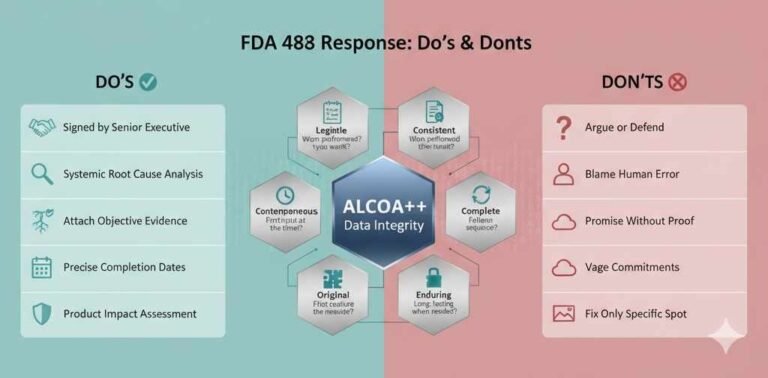
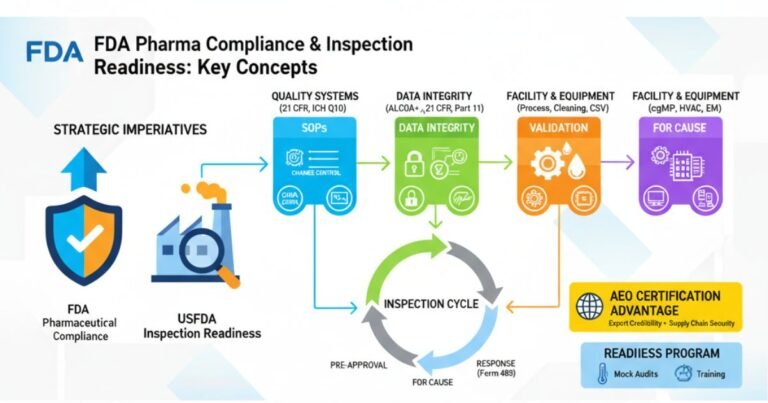
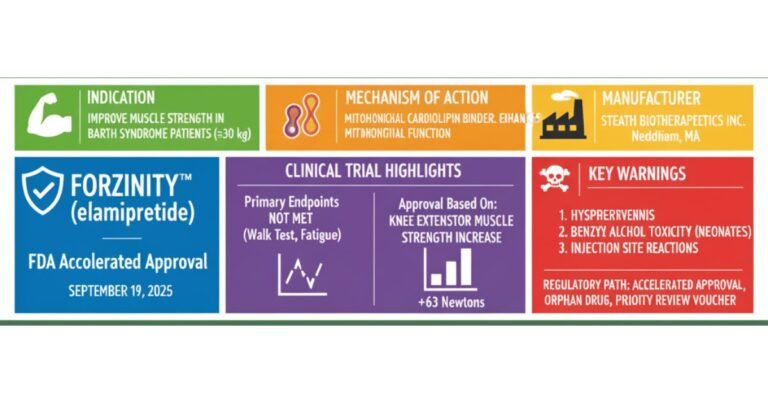
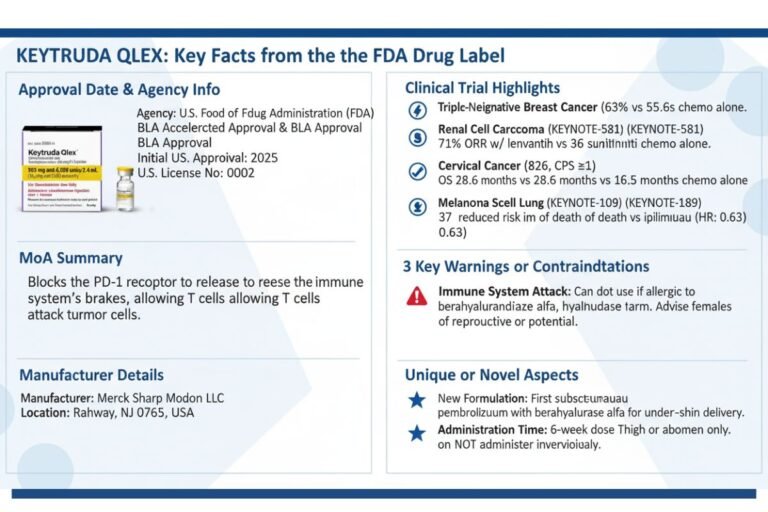
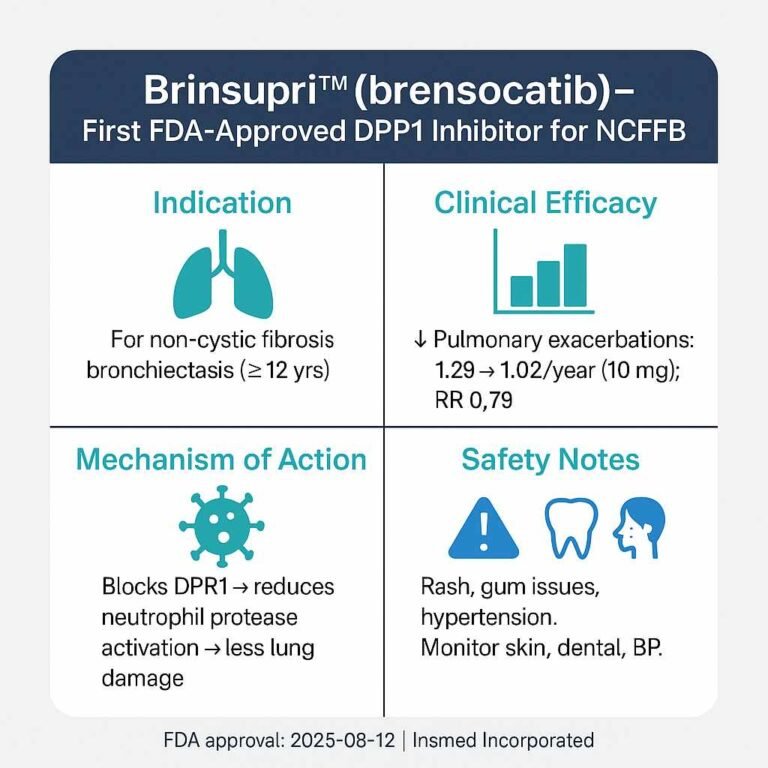
Risk-Based USFDA Inspections ICH Q9 Guide (ICH Q9/QRM 2025 Update)
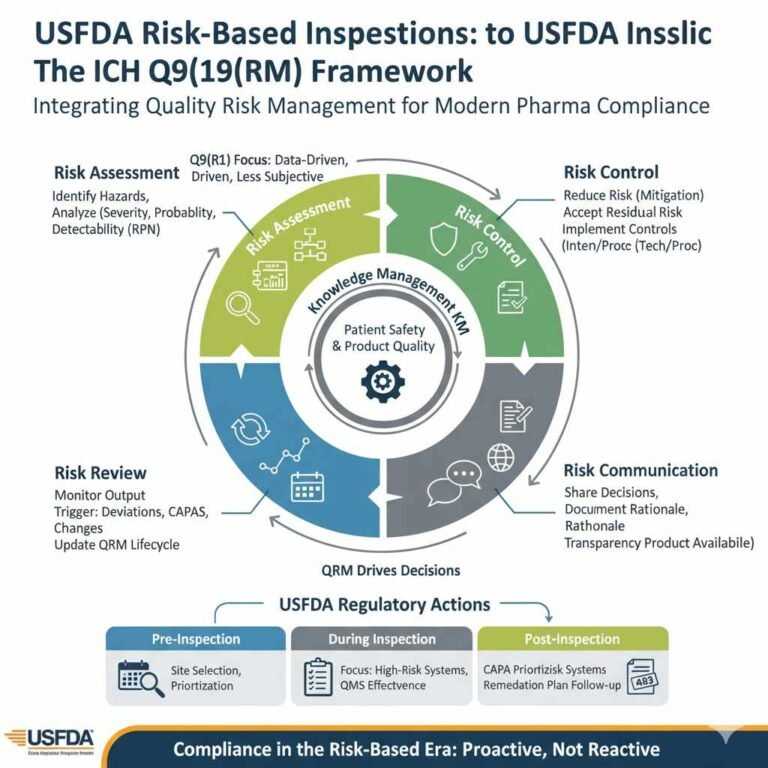
As the pharmaceutical landscape becomes increasingly complex, the US Food and Drug Administration (USFDA) has definitively shifted from a purely reactive, punitive compliance model to a proactive, risk-based oversight framework. This strategic evolution, championed by the principles of ICH Q9…