Starting a pharmaceutical manufacturing plant in India requires a carefully planned capital investment that varies significantly based on plant type, production capacity, and regulatory requirements. A basic domestic-focused tablet or capsule plant requires ₹5-15 crores minimum, while a mid-scale operation targeting domestic and export markets typically needs ₹50-80 crores. For advanced facilities producing injectables or API manufacturing, investments can exceed ₹200 crores.
Understanding these costs and planning your capital structure strategically can improve your payback period and ROI by 30-40%. This comprehensive guide breaks down every cost component, provides a customizable investment calculator, and reveals government incentive programs that can reduce your burden by up to 25%.
Cost Breakdown by Plant Type
1. Small-Scale Tablet & Capsule Plant (₹5-15 Crores)
Best For: Domestic market entry, mono-therapy products, MSME operators
| Cost Component | Estimated Investment (₹ Crores) | % of Total Budget |
|---|---|---|
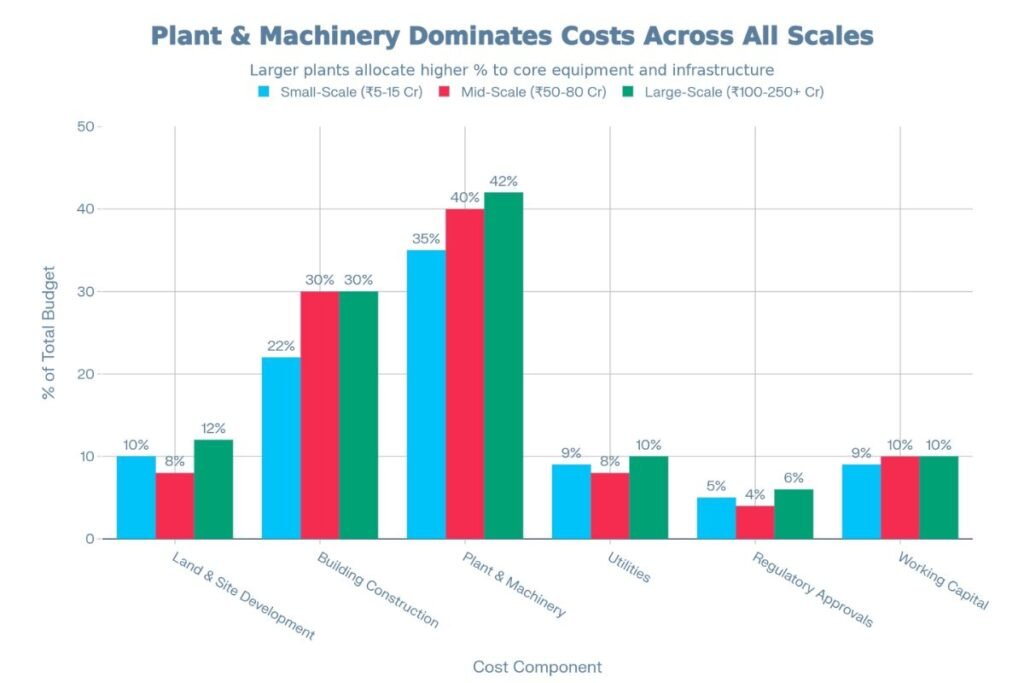
| Land & Site Development | 0.5–2.0 | 8–12% |
| Building Construction | 1.5–4.0 | 18–25% |
| Plant & Machinery | 2.0–5.0 | 30–40% |
| Utilities (HVAC, Water, Power) | 0.5–1.5 | 8–10% |
| Regulatory Approvals & Licensing | 0.3–0.8 | 4–6% |
| Working Capital & Systems | 0.5–1.2 | 7–10% |
| Total CapEx | 5–15 | 100% |
Plant Specifications:
- Built-up area: 2,500–4,000 sq.m
- Production capacity: 20-50 million tablets/month
- Built-in expansion potential: 30-40%
- Payback period: 4–6 years
2. Mid-Scale Plant (₹50-80 Crores)
Best For: Multi-formulation production, export-ready facility, serious scalability
| Cost Component | Estimated Investment (₹ Crores) | % of Total Budget |
|---|---|---|
| Land & Site Development | 4.0–8.0 | 8–10% |
| Building Construction | 15–25 | 25–35% |
| Plant & Machinery | 20–35 | 35–45% |
| Utilities (HVAC, Water, Power, Backup) | 4.0–7.0 | 7–10% |
| Regulatory Approvals, Documentation & QA | 1.5–3.0 | 3–5% |
| Working Capital, Systems & Training | 5–8 | 8–10% |
| Total CapEx | 50–80 | 100% |
Plant Specifications:
- Built-up area: 10,000–15,000 sq.m
- Production capacity: 200+ million tablets/month
- WHO-GMP or international standard compliance
- Payback period: 3–4.5 years
3. Large-Scale / API Manufacturing Plant (₹100-250+ Crores)
Best For: API production, complex formulations (injectables, biologics), export-focused
| Cost Component | Estimated Investment (₹ Crores) | % of Total Budget |
|---|---|---|
| Land & Site Development | 20–60 | 10–15% |
| Building Construction | 50–100 | 25–35% |
| Plant & Machinery | 60–120 | 35–50% |
| Utilities (Captive Power, Water Treatment, Waste Management) | 15–30 | 8–12% |
| Regulatory Approvals, EHS Compliance & QA | 10–20 | 5–8% |
| Working Capital, Manpower Training & Systems | 15–25 | 8–10% |
| Total CapEx | 170–355 | 100% |
Plant Specifications:
- Built-up area: 40,000–80,000 sq.m
- ICH/WHO-GMP compliance (FDA-ready pathway)
- Multi-product capacity: tablets, capsules, injectables
- Payback period: 3–5 years (highly profitable at scale)
Detailed Cost Analysis: Expense-by-Expense Breakdown
Land & Site Development (8–15% of CapEx)
Factors Affecting Cost:
- Urban/Metro Locations: ₹1-3 crore/acre (higher compliance, better logistics)
- Tier-2 Cities: ₹40-80 lakhs/acre (balance of cost and infrastructure)
- Industrial Zones/SEZ: ₹20-40 lakhs/acre (incentive benefits, regulatory support)
For a 5-acre plot in a Tier-2 location:
- Land acquisition: ₹2–4 crores
- Site development (leveling, drainage, boundary wall): ₹50 lakhs–₹1.5 crores
- Total: ₹2.5–5.5 crores
Building Construction (18–35% of CapEx)
Construction Costs: ₹25,000–₹40,000 per sq.m (depending on compliance level)
Key Components:
- Production Block: Cleanrooms (ISO Class 7–8), HVAC systems, antibiotic isolation suites
- QC Laboratory: Advanced instrumentation spaces, microbial testing areas
- Warehouse: Climate-controlled storage for raw materials, finished goods, packaging
- Utility Block: Boiler rooms, water treatment systems, compressed air stations
- Administrative & Training Areas: Offices, training facilities, records management
For a 10,000 sq.m mid-scale facility:
- Construction cost: ₹25–40 per sq.m = ₹2.5–4 crores
- Special installations (HVAC, utilities, cleanrooms): ₹2–4 crores
- Total: ₹4.5–8 crores
Plant & Machinery (30–50% of CapEx)
This is your largest cost driver. Quality here determines long-term reliability and regulatory compliance.
Tablet/Capsule Line (Single Integrated Setup):
- Blender/Granulator: ₹30–50 lakhs
- Tablet/Capsule Filling Machine: ₹50–100 lakhs
- Coating Equipment (if needed): ₹25–50 lakhs
- Packaging Equipment: ₹30–60 lakhs
- Single Production Line Total: ₹1.5–2.5 crores
For a mid-scale 4-line facility: ₹6–10 crores
Cost Optimization Strategies:
- Indian Equipment: 30–40% cheaper than European imports, but verify quality certifications
- Refurbished/Used Machinery: 50–60% savings, requires vendor credibility verification
- Lease Option: ₹20–30 lakhs monthly vs. ₹1.5 crore capital (spreads cost over 5 years)
Utilities & Infrastructure (7–15% of CapEx)
Essential Systems:
- Water Treatment & Supply: ₹50 lakhs–₹1.5 crores (RO systems, storage tanks, distribution)
- HVAC System: ₹1–2 crores (for cleanroom maintenance and personnel comfort)
- Electrical Infrastructure: ₹50–100 lakhs (transformers, backup generators, control panels)
- Backup Power (Diesel Generator): ₹25–50 lakhs (critical for regulated production)
- Waste Treatment System: ₹25–50 lakhs (pharma waste is highly regulated)
Regulatory Approvals & Licensing (3–6% of CapEx)
Upfront Costs (Before Operations):
- Drug Manufacturing License: ₹5–10 lakhs
- Environmental Clearance: ₹5–10 lakhs
- Water & Wastewater Management Approvals: ₹3–5 lakhs
- Fire Safety & Building Compliance: ₹2–3 lakhs
- GST & Labour Law Registration: ₹1–2 lakhs
Ongoing Compliance (Annual):
- License Renewal: ₹3–5 lakhs
- Environmental Audits: ₹3–5 lakhs
- Quality Audits & Inspections: ₹5–10 lakhs
How Location Impacts Your Investment
Regional Cost Variations
| Region | Cost Factor | Land Price/Acre | Labor Monthly | Compliance Ease | Best For |
|---|---|---|---|---|---|
| Tier-1 (Mumbai, Delhi, Bangalore) | 1.4–1.6x | ₹2–3 Cr | ₹18,000–25,000 | Highest | Export/USFDA plants |
| Tier-2 (Ahmedabad, Pune, Hyderabad) | 1.0–1.2x | ₹40–80 L | ₹12,000–18,000 | High | Balanced growth |
| Pharma Clusters (Hyderabad, Ahmedabad) | 0.9–1.1x | ₹30–60 L | ₹10,000–15,000 | Highest | Economies of scale |
| Industrial Zones (SEZ) | 0.8–1.0x | ₹20–40 L | ₹8,000–12,000 | Highest (incentives) | Cost-optimized ops |
| Tier-3 (Emerging hubs) | 0.7–0.9x | ₹10–20 L | ₹6,000–10,000 | Medium | Budget startups |
Strategic Insight: Pharma clusters in Hyderabad, Ahmedabad, and Gujarat provide 15–25% cost savings plus regulatory familiarity and supplier networks.
Operating Costs: Year-1 Onwards (Monthly Recurring)
Once your plant is operational, budget for these monthly expenses:
| Cost Category | Small Plant (₹ Lakhs) | Mid-Scale Plant (₹ Lakhs) | Large Plant (₹ Lakhs) |
|---|---|---|---|
| Raw Materials & Excipients | 8–15 | 35–70 | 150–300 |
| Labor (Direct & Indirect) | 5–10 | 20–40 | 100–200 |
| Utilities (Power, Water, Waste) | 2–4 | 8–15 | 40–80 |
| Packaging Materials | 3–6 | 15–30 | 60–120 |
| Compliance & QA | 1–2 | 5–10 | 30–50 |
| Maintenance & Spares | 1–2 | 5–8 | 20–40 |
| Distribution & Logistics | 2–4 | 10–20 | 50–100 |
| Total Monthly OpEx | 22–43 | 98–193 | 450–890 |
| Monthly Revenue (at 50% capacity) | 20–30 | 100–150 | 500–800 |
Key Insight: Most mid-scale plants reach break-even in 18–24 months and positive cash flow by month 24–30.
Financing Your Pharma Plant Investment
Option 1: Bank Loans
- Eligibility: Registered entity, 20–30% own equity required
- Amount: Up to ₹25 crores for manufacturing plants
- Interest Rate: 6.5–9.5% (floating rate, subject to MSME/industry policy)
- Tenure: 7–10 years
- Collateral: Plant & machinery, land, personal guarantee
- Example: ₹60 crore investment = ₹18 crore own capital + ₹42 crore loan @ 8.5% = ₹4.8 crore/year EMI
Option 2: Government Subsidies & Schemes
- Haryana Pharmaceutical Policy 2019: 25% subsidy on plant & machinery (capped at ₹50 lakhs)
- Production-Linked Incentive (PLI) Scheme: Incentives based on incremental production
- MSME Support: Preferential lending rates, subsidy on ISO certifications
- Technology Upgrade Fund: Support for automation and advanced equipment
Option 3: Venture/Private Equity
- Best For: High-growth, export-focused operations
- Typical Investment: ₹10–50 crores
- Equity Stake: 20–40%
- Value-Add: Industry expertise, market access, operational guidance
- Exit Timeline: 5–7 years
Option 4: Public Sector/Pharma Parks
- Several states (Haryana, Telangana, Gujarat) offer readymade plots with subsidy incentives
- Reduces land acquisition cost by 40–60%
- Pre-built infrastructure (water, power, waste treatment shared)
- Cost Savings Example: ₹5 crore land cost → ₹2–2.5 crores in pharma parks
Real-World Case Scenarios
Case 1: Budget-Conscious Tablet Manufacturer (₹8 Crores)
Goal: Start domestic market operations with growth potential
- Investment Allocation:
- Land (2 acres, Tier-2 city): ₹1.2 Cr
- Building (5,000 sq.m): ₹1.5 Cr
- 2-line tablet setup: ₹2.5 Cr
- Utilities, QC Lab, Packaging: ₹1.3 Cr
- Working capital & contingency: ₹0.5 Cr
- Financing Mix:
- Own capital: ₹2.4 Cr (30%)
- Bank loan: ₹5.6 Cr @ 8.5% = ₹62.5 lakhs/year EMI
- Government subsidy: ₹20 lakhs (if in eligible zone)
- Year-1 Financial Projections:
- Monthly revenue (50% capacity): ₹22 lakhs
- Monthly OpEx: ₹28 lakhs
- Monthly EMI: ₹5.2 lakhs
- Net monthly loss (ramp-up phase): ₹11 lakhs
- Break-even (at full capacity): Month 22–24
Case 2: Scaling Mid-Scale Operation (₹65 Crores)
Goal: Multi-formulation, export-ready facility for institutional buyers
- Investment Allocation:
- Land (5 acres, pharma cluster): ₹2.5 Cr
- Building (12,000 sq.m, WHO-GMP): ₹4 Cr
- 4-line integrated setup: ₹22 Cr
- Utilities, QC, validation systems: ₹6 Cr
- Working capital, training, systems: ₹5 Cr
- Buffer (10%): ₹3 Cr
- Financing Mix:
- Promoter equity: ₹19.5 Cr (30%)
- Bank loan: ₹39 Cr @ 7.8% = ₹4.05 Cr/year EMI
- Pharma park subsidy: ₹6.5 Cr (subsidy on eligible components)
- Year-1 Projections:
- Monthly revenue (70% capacity): ₹75 lakhs
- Monthly OpEx: ₹98 lakhs
- Monthly EMI: ₹33.75 lakhs
- Break-even: Month 28–32
- ROI Stabilization: Year 3–4 (25–30% EBITDA margins)
Government Incentives & Subsidies You Should Leverage
Haryana Pharmaceutical Policy 2019
- Capital Subsidy: 25% of plant & machinery cost (capped at ₹50 lakhs)
- Employment Incentive: ₹5,000 per employee generated (capped at ₹1 crore)
- Interest Subsidy: 4% interest subsidy on bank loans for 5 years
- Land Availability: Pharma parks at ₹15–20 lakhs/acre
Impact: Can reduce your ₹65 Cr investment by ₹15–20 crores
Telangana Pharmaceutical Incentive Scheme
- Special packages for API manufacturers
- ₹10–25 Cr subsidy for large investments
- Land at subsidized rates in dedicated pharma zones
Gujarat Industrial Development Policy
- 8-year tax exemption for new manufacturing units
- Land allocation at minimal rates in pharma clusters
- 50% subsidy on pollution control equipment
PLI Scheme (Pharmaceuticals API)
- Incentive of 5–10% on incremental production and sales
- Applicable to domestic formulations and APIs
- Encourages local manufacturing over imports
ROI & Profitability Timeline
Expected Financial Metrics (Mid-Scale Plant)
Year-by-Year Cash Flow Analysis:
| Metric | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|
| Capacity Utilization | 50–60% | 70–75% | 85–90% | 95%+ | 100% |
| Revenue (₹ Crores) | 8–10 | 13–16 | 22–26 | 32–38 | 40–48 |
| EBITDA Margin | 5–8% | 15–18% | 22–26% | 28–32% | 32–36% |
| CapEx (One-time) | 65 | — | Expansion 5–8 | — | — |
| Payback Period | 3.5–4 years | — | — | — | — |
| Cumulative Net Cash Flow | (55)–(60) | (45)–(50) | (20)–(25) | 5–12 | 25–35 |
Key Insight: The sweet spot for pharma manufacturing is Years 3–5 when capacity utilization hits 85%+ and EBITDA margins climb to 25–35%.
Risk Mitigation: Budgeting for Hidden Costs
- Regulatory Changes (5–10% buffer): License upgrades, compliance retrofits
- Machinery Breakdowns (3–5% buffer): Emergency spare parts, extended warranties
- Working Capital Shortfalls (10% buffer): Extended payment terms to distributors
- Market Delays (2–3% buffer): Ramp-up periods, market education
- Currency Fluctuations (if importing equipment): Consider hedging strategies
Recommendation: Add 12–15% contingency to your total CapEx estimate.
Frequently Asked Questions
What’s the absolute minimum investment to start a pharma plant?
₹3–5 crores for a bare-minimum home/contract manufacturing setup without WHO compliance. However, ₹8–12 crores is realistic for a sustainable, scalable operation.
Can I start with just 1 product line?
Yes, but profitability is lower. Single-line plants achieve 12–15% EBITDA margins; multi-line setups reach 25–35%. Growth to 2–3 lines within 18–24 months is recommended.
Is WHO-GMP certification required from day 1?
No. Start with domestic formulations (basic compliance), then upgrade to WHO-GMP for export markets. This reduces initial CapEx by 15–20% but limits revenue upside initially.
Action Plan: From Today to Operations
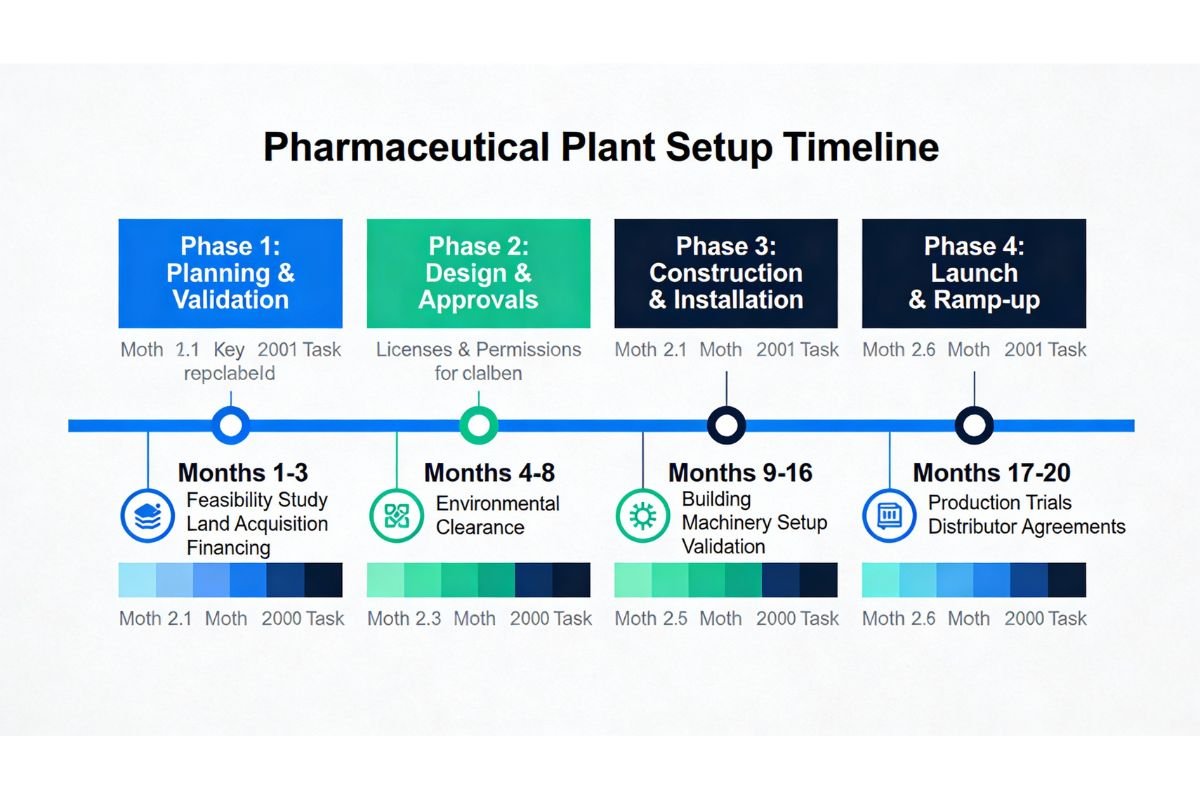
Phase 1: Planning & Validation (Months 1–3)
- Finalize plant type and product profile
- Conduct feasibility study (₹5–10 lakhs investment)
- Identify location and secure land
- Apply for government subsidies (parallel to other tasks)
- Secure financing commitment (bank/investors)
Phase 2: Design & Approvals (Months 4–8)
- Finalize plant layout and equipment specs
- Obtain environmental clearance (2–4 months)
- Secure water/electricity permissions
- Apply for drug manufacturing license (pre-commencement stage)
Phase 3: Construction & Installation (Months 9–16)
- Begin civil construction
- Procure and install machinery
- Set up utilities and waste treatment
- Conduct equipment validation
Phase 4: Launch & Ramp-up (Months 17–20)
- Obtain final drug manufacturing license
- Hire and train workforce
- Conduct production trials
- Secure distributor agreements
- Begin pilot batch sales (Month 18–19)
Conclusion: Your Investment Roadmap
The bottom line: A credible, scalable pharmaceutical manufacturing plant requires ₹8–80 crores depending on your ambitions. The key to profitability is balancing upfront capital investment with operational efficiency and market readiness.
Your next steps:
- Use the interactive calculator above to model your specific scenario
- Assess location options based on land costs and subsidy availability
- Explore financing mixes (30% equity, 70% debt is typical)
- Check government incentive eligibility before finalizing location
- Build a 5-year financial model and stress-test it under conservative demand assumptions
With proper planning, realistic cost budgeting, and strategic use of government incentives, your pharma manufacturing venture can achieve positive cash flow by Year 2–3 and exceptional returns by Year 4–5.
Need Personalized help?
Contact Us
Get a customized cost sheet with real-time cost breakdowns, financing options, and 5-year financial projections for your specific plant type and location.
Additional Resources
- Related: Buy a Running Pharma Company In Himachal Pradesh
- Related: Pharma Facility for Sale Rudrapur
Author & Expertise
This guide is authored by Darshan Singh, a pharmaceutical manufacturing consultant with 23+ years of experience in plant setup, quality assurance, and regulatory compliance. Darshan Singh has guided 40+ pharma startups through the investment-to-operations journey, resulting in ₹500+ crores in capital deployment and 98% on-time project completions.
Last Updated: December 2025 | Data Sources: IMARC Group, NACS India, Government of India Press Releases, FDA & WHO Guidelines
Frequently Asked Questions:
What is the typical timeframe for a pharma plant setup?
From initial planning to commercial launch, a small to medium-scale plant typically takes 1.5 to 2.5 years, with larger or sterile facilities requiring longer.
How important is location for cost?
Extremely. Land costs, labor availability, utility infrastructure, and local incentives vary significantly by region in India, directly impacting your initial investment and ongoing operational costs.
Can I scale up later?
Yes, future scalability should be a key consideration during the facility design & layout phase. Planning for modular expansion can save significant costs and disruption later.
What are the ongoing operational costs beyond initial setup?
Beyond working capital, anticipate continuous expenses for raw material procurement, salaries, utility bills, routine maintenance contracts, annual license renewals, continuous staff training, R&D investments, and ongoing quality assurance audits.
Is government support easily accessible?
Government schemes like PLI are well-structured. However, accessing them requires thorough documentation and adherence to specific criteria. Engaging consultants with expertise in navigating these schemes can be beneficial.
References:
- Business Today
- PIB.GOV.IN/Ministry of Commerce & Industry
- Phrma.org