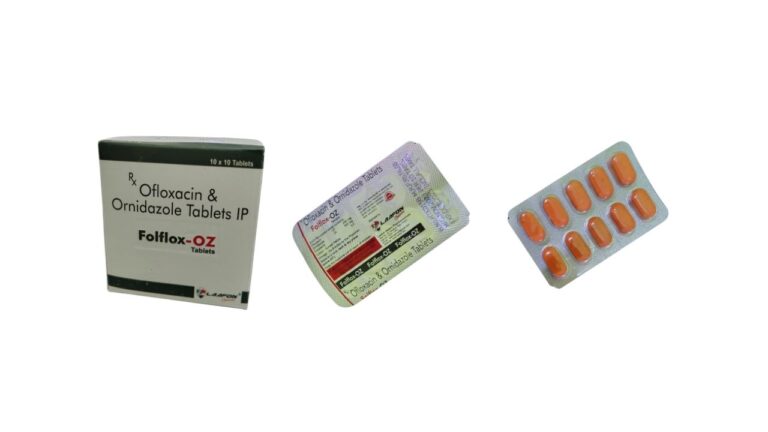
Down-M | Mefenamic acid and Paracetamol suspension

Down-M suspension is the combination of two medicaments Mefenamic Acid and Paracetamol suspension. Mefenamic acid is a member of the anthranilic acid derivatives, a class of nonsteroidal anti-inflammatory drugs. It is used for fever, mild pain, and stomach pain with…