Syrup vs. Suspension: What’s the Real Difference?
In the world of liquid medications, the terms “syrup” and “suspension” are often used interchangeably in casual conversation. However, in pharmaceutical science, syrups and suspensions represent two fundamentally different approaches to delivering medicine. The distinction is critical, impacting everything from how the medicine is made to how it must be used.
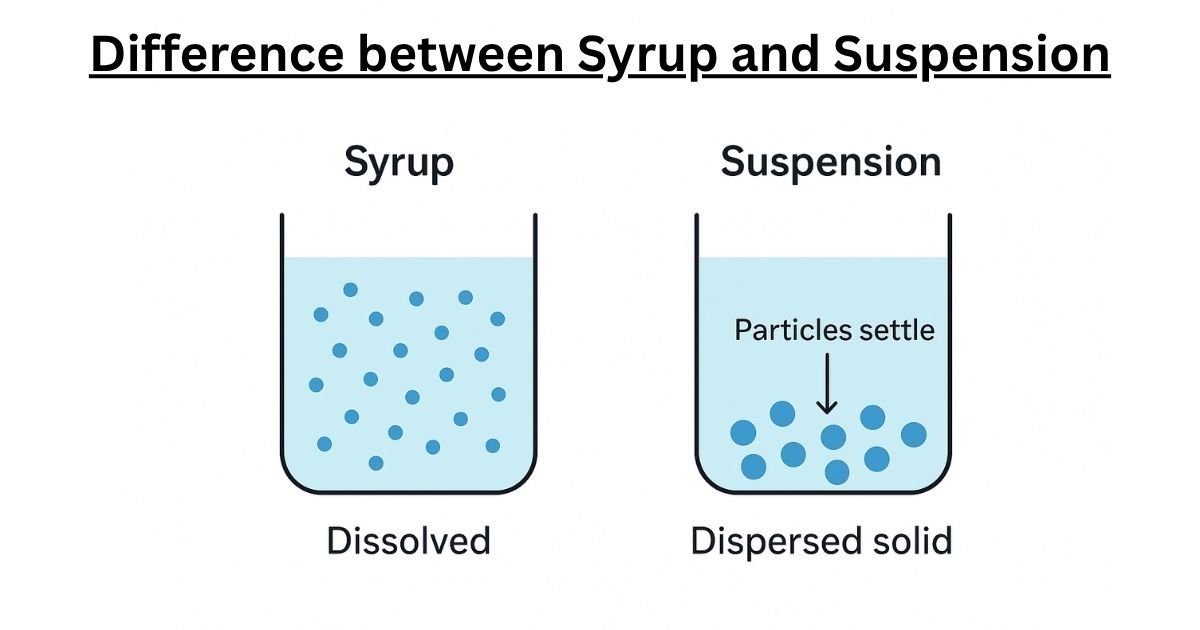
To grasp the core difference, consider a simple kitchen analogy. If you stir a spoonful of sugar into a glass of warm water, the sugar crystals disappear, dissolving completely to create a clear, sweet liquid. Every sip of this water will taste equally sweet because the sugar molecules are evenly distributed. This is a solution, the principle behind a pharmaceutical syrup.
Now, imagine stirring a spoonful of fine sand into another glass of water. The sand particles disperse, making the water cloudy, but they do not dissolve. If left to stand, the sand will slowly settle to the bottom, leaving clear water on top. To get a mouthful of sandy water, you would need to stir it vigorously right before drinking. This is a dispersion, the principle behind a pharmaceutical suspension.
This simple contrast between dissolving and dispersing is the central concept that separates syrups from suspensions, dictating their formulation, stability, and proper use.
Comprehensive Definition of Syrup
A pharmaceutical syrup is a specific type of oral solution. A solution is defined as a homogeneous mixture, meaning its components are perfectly and uniformly blended down to the molecular level. In a syrup, the active pharmaceutical ingredient (API), which is the drug itself, acts as the solute. It is completely dissolved in a liquid vehicle, the solvent.
At the molecular level, “dissolved” means that the individual molecules of the API have broken away from their solid-state crystal structure and are now individually dispersed among the molecules of the solvent. This complete dissolution is why syrups are typically clear and transparent; there are no solid particles large enough to scatter light. Because the drug molecules are evenly distributed throughout the entire volume of the liquid, every dose taken from the bottle—whether it’s the first or the last—contains the exact same concentration of medication. This inherent uniformity means that syrups do not need to be shaken before administration.
While many types of pharmaceutical solutions exist, the term “syrup” specifically denotes a solution that is viscous and sweet. This characteristic is almost always achieved through a high concentration of sugar, such as sucrose, or a sugar substitute. This sweetness is not merely for taste; it is a crucial functional property that helps mask the often unpleasant or bitter taste of the API, making the medication more palatable and improving patient compliance, especially in pediatric and geriatric populations.
Defining Suspensions
In stark contrast, a pharmaceutical suspension is a heterogeneous mixture. This means it consists of at least two physically distinct phases: a solid phase (the insoluble API particles) dispersed throughout a liquid phase (the vehicle or continuous phase).
The defining characteristic of a suspension is the insolubility or poor solubility of the API in the liquid vehicle. The drug does not dissolve into individual molecules. Instead, it exists as microscopic solid particles, typically ranging from 0.5 to 200 micrometers in diameter, that are simply spread throughout the liquid. Because these particles are much larger than molecules, they scatter light, which is why suspensions appear cloudy or opaque.
The choice to create a suspension is not arbitrary; it is a necessity dictated by the fundamental physicochemical properties of the drug. If an API is not soluble in water or other safe-to-ingest solvents, it cannot be formulated as a solution or syrup. A suspension therefore becomes the required liquid dosage form.
This state of dispersion is inherently unstable. The solid drug particles are almost always denser than the liquid they are in, making them susceptible to the force of gravity. Over time, these particles will inevitably settle to the bottom of the container, a process known as sedimentation. This is the fundamental reason why every suspension is labeled with the critical instruction:
“Shake Well Before Use.” Shaking is essential to resuspend the settled particles, ensuring a uniform distribution throughout the liquid so that an accurate and consistent dose can be administered.
A Detailed Comparison in Syrup and Suspension
For those seeking a quick and clear summary, the following table distills the essential differences between these two common liquid dosage forms. This comparison serves as a high-level overview for healthcare professionals and a straightforward guide for patients and caregivers.
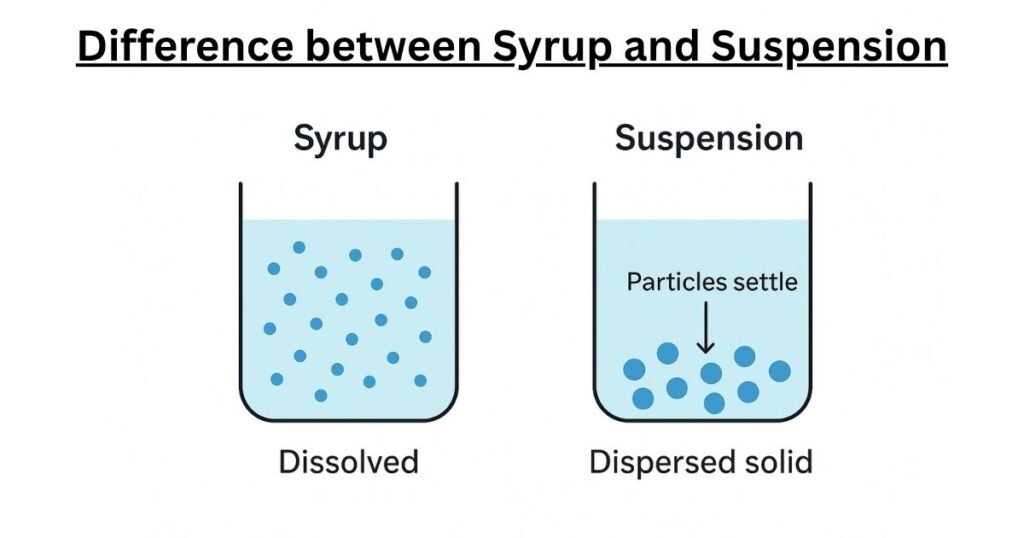
Table 1: Syrup vs. Suspension – Key Differences
| Attribute | Syrup (A Type of Solution) | Suspension (A Type of Dispersion) |
| Physical State | Homogeneous mixture (one phase) | Heterogeneous mixture (two phases: solid in liquid) |
| Appearance | Clear, transparent | Cloudy, opaque |
| API State | Active Pharmaceutical Ingredient (API) is fully dissolved | API exists as insoluble, solid particles |
| Particle Size | Molecular level (individual molecules) | Microscopic particles (e.g., 0.5-200 µm) |
| Homogeneity | Uniform throughout the liquid; consistent concentration | Non-uniform if left standing; concentration varies with depth |
| Need to Shake | No, the drug remains evenly distributed | Yes, absolutely critical. Must be shaken well before every dose |
| Viscosity | Typically high and thick due to concentrated sugar | Highly variable; can be formulated to be thick or thin |
| Taste Profile | Generally sweet and palatable to mask API taste | Taste masking is more challenging; may be less pleasant |
| Primary Formulation Goal | To completely dissolve the API in a stable, palatable vehicle | To keep insoluble particles uniformly suspended and easily redispersible |
| Dosage Accuracy | High and consistent with each dose | Can be highly inaccurate if not shaken properly |
| Primary Stability Concern | Microbial growth (if not properly preserved); chemical degradation of the dissolved API; crystallization of sugar | Physical instability: particle settling (sedimentation), irreversible caking, and crystal growth |
| Common Examples | Cough medicines (Guaifenesin, Dextromethorphan), some antihistamines, simple syrups for compounding | Many children’s antibiotics (Amoxicillin, Azithromycin), antacids, Nystatin antifungal, some anti-inflammatory drugs |
Importance and Science behind Syrups
The apparent simplicity of a syrup—a drug dissolved in sugar water—belies the complex formulation science required to create a product that is stable, effective, and palatable. Each ingredient is carefully selected to perform multiple, often overlapping, functions. The formulation of a syrup is a delicate balancing act, where the choice of one component has cascading effects on all others.
Science of Syrups Formulation
A pharmaceutical syrup is composed of several key ingredients, each with a specific purpose :
- Active Pharmaceutical Ingredient (API): This is the therapeutic substance. The primary prerequisite for a syrup formulation is that the API must be soluble in the chosen aqueous vehicle.
- Vehicle/Solvent: The liquid base of the formulation is almost always purified water. In cases where the API has limited water solubility, co-solvents such as ethanol, glycerin, or propylene glycol may be added. These agents not only help dissolve the API but can also function as preservatives and viscosity enhancers.
- Sweeteners: This is the defining component of a syrup.
- Sucrose: This is the traditional and most common sweetener, prized for its purity, pleasant taste, and lack of color. Pharmaceutical syrups often contain very high concentrations of sucrose, typically 60% to 85% by weight. For example, Syrup, USP, a standard pharmacopeial formula, contains 85 grams of sucrose for every 100 mL of syrup. This high concentration serves three purposes: it provides intense sweetness, creates the desired high viscosity, and imparts a preservative effect by reducing the amount of “free” water available for microbial growth.
- Sugar Substitutes: For patients who must limit their sugar intake, such as those with diabetes, alternative sweeteners are used. These fall into two categories:
- Polyols (Sugar Alcohols): Sorbitol, mannitol, and glycerin are common choices. They provide sweetness and contribute to the syrup’s viscosity. A 70% sorbitol solution is a widely used commercial vehicle.
- Non-Nutritive Sweeteners: Artificial sweeteners like aspartame or saccharin provide sweetness without calories. However, they do not contribute to viscosity, so formulations using them require the addition of a separate thickening agent, such as methylcellulose, to achieve the desired syrupy texture.
- Preservatives: While highly concentrated sucrose syrups are self-preserving, more dilute syrups are excellent media for the growth of bacteria, yeasts, and molds. In these cases, or as an added safeguard, antimicrobial preservatives are essential. Common examples include benzoic acid, sodium benzoate, and various parabens. The amount of preservative needed is calculated based on the volume of “free water”—the water not osmotically bound by sucrose—that is susceptible to microbial contamination.
- Flavorants and Colorants: To enhance patient acceptance, especially for pediatric formulations, a wide variety of natural and artificial flavors and colors are used. These agents mask the API’s taste and provide a consistent, appealing appearance.
- Buffers: Some APIs are only stable within a narrow pH range. Buffering agents, such as citrate or phosphate systems, are added to maintain the pH of the syrup and prevent the degradation of the drug.
Manufacturing Processes: From Raw Materials to Finished Product
The method chosen to manufacture a syrup depends primarily on the chemical and physical characteristics of its ingredients, particularly their sensitivity to heat.
- Solution with Heat: This is the fastest and most common method for dissolving large quantities of sucrose. The ingredients are mixed in water and heated to facilitate dissolution. However, this method is unsuitable for syrups containing heat-sensitive (thermolabile) APIs or volatile flavoring agents, as the heat can cause them to degrade or evaporate. Careful temperature control is crucial to prevent the overheating of sucrose, which can lead to hydrolysis (breaking down into glucose and fructose) and caramelization, a process that imparts an undesirable dark color to the syrup.
- Agitation without Heat: To avoid heat-related degradation, syrups can be prepared at ambient temperature using vigorous agitation. This process is much slower and requires more mechanical energy to achieve complete dissolution. Modern manufacturing facilities often employ high-shear mixers, which can create intense turbulence to rapidly dissolve sugars and other powders without the need for external heating, thereby protecting sensitive ingredients and saving energy.
- Percolation: In this specialized method, purified water or an aqueous solution is slowly passed through a column (a percolator) packed with crystalline sucrose or other ingredients. The liquid dissolves the ingredients as it flows through the column, emerging as a finished or nearly finished syrup. This method is particularly useful for extracting active ingredients from vegetable materials, as in the preparation of Ipecac Syrup.
Key Challenges in Syrup Formulation and Manufacturing
Despite their apparent simplicity, syrups present unique challenges that require careful management:
- High Viscosity: The thick, viscous nature of syrups, while desirable for mouthfeel and suspending properties, can complicate manufacturing. Highly viscous liquids are difficult to pump, filter, and fill into bottles accurately and efficiently.
- Crystallization and “Cap Locking”: In highly concentrated sucrose syrups, temperature fluctuations can cause the sugar to crystallize out of solution. These crystals often form on the threads of the bottle neck and cap, effectively cementing the cap shut—a phenomenon known as “cap locking.” The inclusion of polyols like glycerin or sorbitol in the formulation can help prevent this by interfering with sucrose crystallization.
- Microbial Stability: As aqueous preparations, syrups are inherently susceptible to microbial contamination. Ensuring the preservative system is robust and effective throughout the product’s shelf life is a primary concern for formulators and a key focus of regulatory inspections.
- “Salting Out”: The high concentration of sucrose in a syrup ties up a large number of water molecules through hydrogen bonding. This reduces the amount of water available to dissolve other substances, including the API. This can lead to a phenomenon called “salting out,” where the API precipitates out of the solution. Formulators must carefully assess the solubility of the API in the final, highly concentrated syrup vehicle, not just in plain water.
The Art and Science of Suspensions
Formulating a pharmaceutical suspension is a far more complex endeavor than formulating a syrup. It is an exercise in controlled physical chemistry, where the goal is not to achieve perfect stability—which is thermodynamically impossible for a dispersion—but to engineer a state of controlled instability. The final product must be a dynamic system that is safe, effective, and predictable, capable of being returned to its functional state by the simple act of shaking.
The Core Components of Suspension
The creation of a successful suspension relies on a synergistic combination of its core components:
- Active Pharmaceutical Ingredient (API): The drug must be insoluble or poorly soluble in the vehicle. Unlike in a syrup, the physical characteristics of the solid API are of paramount importance. Properties such as particle size, particle size distribution, crystal shape (habit), density, and surface charge are critical variables that the formulator must understand and control, as they directly influence the suspension’s stability and bioavailability.
- Vehicle (Continuous Phase): This is the liquid in which the API particles are dispersed. Purified water is the most common vehicle for oral suspensions. For non-oral applications, such as topical or injectable products, oils or other organic liquids may be used.
- Excipients: These are the functional ingredients—the “magic” in the formulation—that are used to manage the inherent instability of the system. Mastering their use is the key to successful suspension design.
Essential Excipients for Suspensions
To transform an insoluble powder into a stable and effective medication, formulators rely on a specialized toolkit of excipients.
Dissolving Agents for Suspension
- Function: This is the crucial first step in making a suspension. Many drug powders are hydrophobic (water-repelling). When added to water, they tend to clump together and float on the surface, trapped by a layer of air, making uniform dispersion impossible. Wetting agents are surfactants that reduce the interfacial tension between the solid particle and the liquid vehicle. They act like a bridge, allowing the water to displace the air, surround the particle, and “wet” it completely.
- Examples: Common wetting agents include polysorbates (e.g., Polysorbate 80, also known as Tween 80), sorbitan esters, and sodium lauryl sulfate (SLS). Co-solvents with surfactant-like properties, such as glycerin and propylene glycol, are also frequently used.
Suspending Agents (Viscosity Modifiers)
- Function: Once the particles are wetted, the next challenge is to keep them from settling too quickly. Suspending agents, also known as thickeners, increase the viscosity of the continuous phase. A more viscous liquid offers greater resistance to the movement of particles, thereby slowing down the rate of sedimentation. The ideal suspending agent creates a shear-thinning (pseudoplastic or thixotropic) system: the suspension is thick and gel-like at rest to hold the particles up, but becomes thin and flows easily when agitated (shaken), allowing for accurate pouring and dosing.
- Examples:
- Natural Polysaccharides (Gums): Acacia, Tragacanth, and especially Xanthan Gum are widely used for their excellent suspending and shear-thinning properties.
- Cellulose Derivatives: This is a large and versatile class, including Methylcellulose (MC), Sodium Carboxymethylcellulose (NaCMC), Hydroxypropyl Methylcellulose (HPMC), and Microcrystalline Cellulose (MCC). MCC is often used in combination with NaCMC (e.g., Avicel® RC-591) to form a robust, thixotropic gel network.
- Clays: Natural mineral clays like Bentonite and Magnesium Aluminum Silicate (e.g., Veegum®) are effective because they hydrate to form three-dimensional colloidal structures that entrap the drug particles.
- Synthetic Polymers: Carbomers (e.g., Carbopol®) are high-molecular-weight polymers of acrylic acid that are highly efficient at creating high-viscosity gels at very low concentrations.
Flocculating Agents
- Function: This is perhaps the most counter-intuitive yet critical aspect of suspension design. Flocculating agents are used to intentionally encourage the individual drug particles to form loose, porous aggregates called “flocs.” While this may seem to defeat the purpose of dispersing the particles, it is a key strategy to prevent the most serious type of physical instability: caking. Flocs, being larger than individual particles, settle more rapidly, but they form a high-volume, “fluffy” sediment that does not pack tightly and is very easily redispersed with gentle shaking. This ensures that the patient can always restore the suspension to a uniform state.
- Examples: The flocculating effect is achieved by carefully controlling the electrostatic forces between particles. This is typically done using electrolytes (e.g., sodium citrate, potassium phosphate, aluminum chloride), surfactants, or certain polymers that can bridge particles together. The choice and concentration of the flocculating agent are critical; too little will not prevent caking, and too much can cause the flocs to become too dense and difficult to redisperse.
Other Excipients used in Suspensions
Suspensions also contain many of the same excipients found in syrups, such as buffers to control pH, preservatives to prevent microbial growth, and sweeteners and flavors to improve palatability.
Table 2: Common Excipients in Liquid Dosage Forms
| Dosage Form | Excipient Category | Primary Function(s) | Specific Examples |
| Syrup | Sweetener / Viscosity Agent | Provides sweetness, viscosity, and can act as a preservative. | Sucrose, Sorbitol Solution (70%), Glycerin, Mannitol, Agave Syrup |
| Syrup | Non-Nutritive Sweetener | Provides sweetness without calories (for diabetic formulations). | Aspartame, Saccharin, Sucralose |
| Syrup / Suspension | Solvent / Co-Solvent | Dissolves API (syrup) or aids in wetting (suspension); can act as a preservative. | Purified Water, Ethanol, Propylene Glycol, Glycerin |
| Syrup / Suspension | Preservative | Prevents microbial growth in the aqueous vehicle. | Sodium Benzoate, Benzoic Acid, Methylparaben, Propylparaben, Potassium Sorbate |
| Suspension | Wetting Agent | Reduces interfacial tension to allow the liquid to displace air and wet the solid API particles. | Polysorbate 80 (Tween 80), Sorbitan Monolaurate (Span 20), Sodium Lauryl Sulfate (SLS) |
| Suspension | Suspending Agent (Gums) | Increases viscosity, slows sedimentation, often provides shear-thinning properties. | Xanthan Gum, Acacia, Tragacanth, Guar Gum |
| Suspension | Suspending Agent (Celluloses) | Forms viscous solutions or gel networks to suspend particles. | Methylcellulose (MC), Sodium Carboxymethylcellulose (NaCMC), Hydroxypropyl Methylcellulose (HPMC), Microcrystalline Cellulose (MCC, Avicel®) |
| Suspension | Suspending Agent (Clays) | Forms a three-dimensional colloidal structure to entrap particles. | Bentonite, Magnesium Aluminum Silicate (Veegum®) |
| Suspension | Suspending Agent (Polymers) | Highly efficient thickener, forms high-viscosity gels. | Carbomer (Carbopol® 974P NF) |
| Suspension | Flocculating Agent | Controls particle aggregation to form loose flocs, preventing caking. | Electrolytes (Sodium Citrate, Aluminum Chloride), Surfactants, Polymers |
| Syrup / Suspension | Buffer | Maintains a stable pH to ensure API stability and/or solubility. | Citric Acid/Sodium Citrate, Phosphoric Acid/Sodium Phosphate |
The Stability Challenge: Why Suspensions Are Inherently Unstable
The core challenge in suspension formulation is a constant battle against physics. A suspension is a thermodynamically unstable system that will always try to minimize its energy by reducing the total surface area of the dispersed particles—meaning the particles will try to aggregate, and gravity will pull them down. The formulator’s job is to use the chemical tools of excipients to manage these physical forces and create a product that remains safe and effective over its entire shelf life.
Understanding Sedimentation of Suspension
The rate at which particles settle in a liquid is described by Stokes’ Law. While the equation applies perfectly only to idealized, non-interacting spherical particles, it provides an invaluable qualitative guide to the factors a formulator can manipulate to control sedimentation. The equation is:
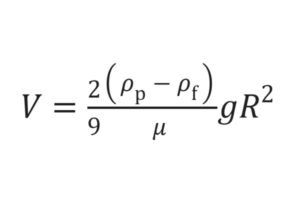
Where:
vis the sedimentation velocity (the speed at which particles settle).dis the diameter of the particle.ρpis the density of the particle.ρfis the density of the liquid medium.gis the acceleration due to gravity.η(eta) is the viscosity of the liquid medium.
Deconstructing the factors reveals the formulator’s strategy:
- Particle Size (
d): This is the most powerful variable. The settling velocity is proportional to the square of the particle diameter. This means that reducing the particle size by half reduces the settling rate by a factor of four. This is why APIs for suspensions are often micronized to a very fine powder. - Density Difference (
ρp - ρf): The closer the density of the liquid is to the density of the particles, the slower the settling. While the particle density is fixed, the formulator can increase the density of the liquid medium by dissolving substances like sorbitol, glycerin, or sucrose in it. This strategy is known as density matching. - Viscosity of the Medium (
η): The settling velocity is inversely proportional to the viscosity. Doubling the viscosity of the liquid cuts the settling rate in half. This is the primary function of suspending agents like xanthan gum or methylcellulose, which dramatically increase the viscosity of the water phase.
The Critical Trade-Off: Flocculated vs. Deflocculated Systems
The most sophisticated aspect of suspension design lies in managing the interactions between the particles themselves. This leads to a critical choice between two types of systems, each with its own stability profile.
- Deflocculated Systems: In this state, the individual particles repel each other and remain as discrete, separate entities. This is achieved by ensuring the particles have a strong surface charge, creating electrostatic repulsion. On the surface, this seems ideal: the particles are fine and settle very slowly. However, this slow settling is deceptive and dangerous. As the particles eventually settle, their mutual repulsion allows them to slide past one another and pack into a dense, ordered arrangement at the bottom of the container. Over time, the pressure from the particles above squeezes out the liquid, and the particles can fuse together, forming a hard, non-redispersible sediment known as a cake. Caking is the ultimate failure of a suspension, as the correct dose can no longer be withdrawn from the bottle.
- Flocculated Systems: In this state, the formulator deliberately reduces the repulsive forces between particles, allowing them to come together and form loose, lightweight aggregates called flocs. This is done by adding a controlled amount of a flocculating agent (e.g., an electrolyte). These flocs behave like larger particles and thus settle much more quickly than individual deflocculated particles. While this rapid sedimentation may seem like a sign of instability, it is actually the key to a safe and stable product. The flocs form a porous, high-volume sediment that does not pack tightly and traps a large amount of the liquid medium within its structure. This “fluffy” sediment is easily broken apart and redispersed into a uniform suspension with gentle shaking.
The goal of modern suspension formulation is therefore to create a system of controlled flocculation, balancing the system so that the sediment is easy to redisperse while ensuring the settling is not so rapid that it prevents accurate dosing.
Table 3: Flocculated vs. Deflocculated Suspensions – A Stability Comparison
| Attribute | Deflocculated System | Flocculated System |
| Particle State | Discrete, separate individual particles | Loose, porous aggregates (flocs) |
| Interparticle Forces | Repulsive forces dominate | Attractive (van der Waals) and repulsive forces are balanced |
| Sedimentation Rate | Slow | Fast |
| Sediment Appearance | Dense, compact, low-volume | High-volume, “fluffy,” porous |
| Supernatant Appearance | Remains cloudy or turbid for a long time | Clears rapidly, distinct boundary with sediment |
| Caking Risk | High. This is the primary failure mode. | Low to None. Flocculation is the primary strategy to prevent caking. |
| Redispersibility | Poor to impossible once a cake has formed. | Excellent. Easily redispersed with gentle shaking. |
| Aesthetic vs. Functional | Initially looks more stable and uniform (pleasing) but is functionally dangerous. | Initially looks less stable (unpleasing) but is functionally superior and safe. |
The Ultimate Failures: Caking and Crystal Growth
Beyond sedimentation, two other phenomena represent critical stability failures in suspensions:
- Caking: As described above, caking is the formation of a non-redispersible sediment. It is the result of particle growth and fusion in a tightly packed sediment bed, often exacerbated by temperature fluctuations. Once a suspension has caked, it is unusable and must be discarded. The prevention of caking through controlled flocculation is therefore a primary objective of the formulation scientist.
- Crystal Growth (Ostwald Ripening): This is a more subtle, long-term instability that affects the particle size distribution of the suspension over its shelf life. Due to normal temperature fluctuations during shipping and storage, a small amount of the API can dissolve into the liquid vehicle. As the temperature cools, this dissolved material will re-precipitate, but it preferentially deposits onto the surface of the larger existing crystals rather than forming new small ones. The net effect is that the small particles get smaller (and eventually disappear) while the large particles get larger. This phenomenon, known as Ostwald ripening, leads to an increase in the average particle size over time. A change in particle size can alter the drug’s dissolution rate, bioavailability, and the suspension’s overall physical stability, potentially compromising the product’s efficacy and safety. Controlling the crystal form of the API and minimizing its solubility in the vehicle are key strategies to mitigate this risk.
From Lab to Pharmacy: Manufacturing, Quality, and Regulation
The journey of a liquid medication from a concept in a formulation lab to a bottle on a pharmacy shelf is fraught with challenges, particularly for complex systems like suspensions. Successfully manufacturing these products at a large scale requires a deep understanding of process engineering, rigorous quality control, and adherence to strict regulatory standards. The manufacturing process is, in essence, the practical application of the formulation science, and the quality control tests are the verification that the science has been successfully implemented.
The Scale-Up Challenge: Why Bigger Isn’t Always Better
Transferring a formulation from a small laboratory batch (a few liters) to full commercial production (hundreds or thousands of liters) is one of the most difficult steps in pharmaceutical manufacturing. This “scale-up” process is far more complex than simply using a bigger mixing tank.
- Process Variables and Equipment Differences: The parameters that create a perfect suspension in a lab-scale mixer—such as mixing speed, time, and temperature—do not translate linearly to large-scale equipment. The hydrodynamics, shear forces, and heat transfer characteristics of a 1,000-liter tank are vastly different from those of a 1-liter beaker. Transferring a process from one piece of equipment to another, even of the same type, often requires extensive experimentation and re-validation to achieve the same product characteristics.
- Achieving Homogeneity: Ensuring that billions of tiny API particles are uniformly dispersed throughout a massive volume of liquid is a significant engineering challenge. “Dead spots” in large tanks where mixing is inefficient can lead to settling. Long transfer lines between the manufacturing tank and the filling line are another potential source of segregation, where particles can settle out before the product even reaches the bottle. This necessitates continuous, gentle agitation throughout the entire process, right up to the point of filling.
- Raw Material Variability: The physical properties of the API powder itself can vary from batch to batch from the supplier. Subtle changes in crystal habit or particle size distribution can have a dramatic effect on the behavior of the final suspension. This requires pharmaceutical manufacturers to have very tight specifications for their raw materials and a close working relationship with their API suppliers.
Quality Control: Ensuring Safety and Efficacy
To ensure that every batch of medication is safe, effective, and consistent, manufacturers perform a battery of quality control (QC) tests on both the raw materials and the final product. These tests measure the Critical Quality Attributes (CQAs)—the physical, chemical, and biological properties that define the product’s quality. For suspensions, these tests directly reflect the parameters that were critical during formulation:
- Particle Size Distribution: This is monitored using techniques like laser diffraction to ensure the API particles are within the specified size range. This is crucial for stability, dissolution, and bioavailability.
- Viscosity (Rheology): A viscometer is used to measure the flow properties of the suspension. This confirms that the suspending agents are working correctly and that the product will have the right consistency for both stability and pourability.
- Zeta Potential: This measurement quantifies the electrical charge on the surface of the dispersed particles. It is a key indicator of the system’s flocculation state and is used to predict long-term stability against aggregation and caking.
- Sedimentation Volume and Redispersibility: These are practical performance tests. Sedimentation volume measures the ratio of the final sediment volume to the original suspension volume, providing a quantitative measure of the “fluffiness” of the sediment. Redispersibility is often tested by placing the bottle on a mechanical shaker and counting the number of inversions required to achieve a uniform appearance. This ensures the patient can easily resuspend the product.
- Assay and Purity: Standard chemical tests (like High-Performance Liquid Chromatography, HPLC) are used to confirm that the product contains the correct amount of API and to detect any impurities or degradation products.
- Microbial Limits: Because they are aqueous, oral liquids are tested to ensure they are free from harmful levels of microorganisms. The presence of certain bacteria, such as Pseudomonas species in antacids, is considered particularly objectionable.
The Regulatory Perspective: FDA Oversight
The U.S. Food and Drug Administration (FDA) provides stringent guidelines for the manufacture of all pharmaceutical products, with a particular focus on oral liquids due to their complexity and the vulnerable populations (pediatric and geriatric patients) that often use them. FDA inspection guides highlight several key areas of concern:
- Microbiological Contamination: This is a major issue. The FDA scrutinizes a facility’s water purification systems, equipment cleaning and sanitization procedures, and preservative systems to prevent contamination. The design of manufacturing equipment, such as the use of flush valves and properly stored hoses, is critical to eliminate areas where microbes can grow.
- Process Control and Validation: Manufacturers must prove that their production process is robust and consistently produces a quality product. This includes having validated time limits for holding bulk suspensions before filling to prevent settling and segregation.
- Facility Design: The physical plant must be designed to prevent cross-contamination between different products, especially when potent or highly sensitizing drugs like steroids or sulfa drugs are involved. This includes having adequate HVAC systems and dust control measures.
Ultimately, the entire chain of activities—from the fundamental science of Stokes’ Law, to the chemical selection of excipients, to the engineering of manufacturing processes, and finally to the rigor of QC testing and regulatory compliance—is interconnected. A failure at any point in this chain can compromise the final product. This intricate web of dependencies underscores why a deep scientific understanding is not just academic but is essential for the successful production of safe and effective liquid medicines.
Practical Guidance for Patients, Caregivers, and Pharmacists
While the scientific and manufacturing complexities of syrups and suspensions are fascinating, the most important information for the end-user revolves around their safe and effective use. Understanding the “why” behind the instructions on a medicine bottle can empower patients and caregivers to become active partners in their own healthcare.
Choosing the Right Formulation: When and Why?
The decision to prescribe a syrup versus a suspension is primarily a clinical and pharmaceutical one, based on the properties of the drug and the needs of the patient.
- When Syrups are Preferred:
- Soluble Drugs: A syrup is the formulation of choice when the API is readily soluble in water.
- Rapid Onset of Action: Because the drug is already dissolved, it can be absorbed more quickly by the body.
- Superior Taste-Masking: The high sugar content and uniform nature of syrups make them exceptionally good at masking unpleasant tastes, a critical factor for patient compliance, especially with children.
- When Suspensions are Necessary or Preferred:
- Insoluble Drugs: If a drug is poorly soluble in water, a suspension is often the only viable liquid dosage form.
- Improved Chemical Stability: Some drugs are chemically unstable when they are in solution but are quite stable in their solid, crystalline form. Formulating them as a suspension can significantly extend their shelf life. This is a key reason many antibiotics for reconstitution are supplied as powders.
- Sustained Release: For some drugs, a slower, more controlled release is desired. In a suspension, the drug must first dissolve in the gastrointestinal fluids before it can be absorbed, which can prolong its action.
- Patients with Dysphagia: For any patient who has difficulty swallowing pills or capsules (dysphagia), including infants, children, and the elderly, a liquid form like a suspension is a critical alternative.
Proper Administration, Dosing, and Storage
Correctly administering liquid medications is crucial for safety and efficacy.
- The Critical “Shake Well” Instruction: This is the single most important instruction for any suspension. It is not a suggestion. Failure to shake the bottle vigorously before every single dose will result in an incorrect amount of medication being given. The initial doses from an unshaken bottle will be weak (under-dosing), and the final doses will be dangerously concentrated (over-dosing).Syrups, being true solutions, do not need to be shaken.
- Accurate Measurement: Never use household teaspoons or tablespoons to measure medication. These utensils are not standardized and can lead to significant dosing errors. Always use the specific measuring device provided with the medication, whether it is a marked oral syringe, a dropper, or a medicine cup. These are calibrated to deliver an accurate dose.
- Storage: Always follow the storage instructions on the label. Most liquid medications should be stored in a tightly closed container at room temperature, away from direct sunlight and moisture (e.g., not in a bathroom medicine cabinet). Some suspensions, particularly reconstituted antibiotics, require refrigeration to maintain stability. However, many suspensions should not be refrigerated, as cold temperatures can alter their viscosity or even cause them to freeze. Always check the label.
Patient Counseling: A Pharmacist’s Checklist
Effective communication between pharmacists and patients/caregivers is essential to prevent medication errors. The following checklist can guide this crucial conversation, particularly for oral suspensions.
Table 4: Patient Counseling Checklist for Oral Suspensions
| Counseling Point | Key Information to Convey |
| 1. Identify the Medication | “This medicine is called. It’s a suspension, which means the medicine is made of tiny solid particles floating in a liquid. It is used to treat [Condition].” |
| 2. Explain the “Shake Well” Imperative | “This is the most important step. Because the particles settle to the bottom, you must shake the bottle vigorously for at least 10-15 seconds right before you measure out every single dose. If you don’t, the dose will be wrong.” |
| 3. Demonstrate Proper Dosing | “Always use the oral syringe (or cup) that came with this medicine. Do not use a kitchen spoon. Your dose is exactly [X] milliliters (mL). Show them the mark on the syringe/cup.” |
| 4. Review Administration Technique | “For a child, aim the syringe toward the inside of their cheek, not the back of the throat, and give the medicine slowly to prevent choking or spitting.” “This medicine should be taken [with food / on an empty stomach].” |
| 5. Discuss the Full Course of Treatment | “Even if you (or your child) start to feel better, you must finish the entire course of this medication as prescribed by your doctor to ensure the infection is fully treated.” |
| 6. Clarify Storage Requirements | “Store this bottle at [room temperature / in the refrigerator]. Keep it away from direct light. Do not freeze it. Note the expiration date—especially for reconstituted antibiotics, which are often only good for 7-14 days.” |
| 7. Cover Key Side Effects | “Common side effects you might notice are [list 1-2 common, manageable effects like diarrhea or upset stomach]. If you experience any of these serious effects, such as [list 1-2 critical effects like severe rash or difficulty breathing], stop the medicine and contact your doctor immediately.” |
| 8. Check for Understanding | “To make sure I’ve explained everything clearly, can you tell me the two most important things you need to remember when giving this medicine?” (Listen for “shake well” and “use the right syringe”). |
Common Examples in the Pharmacy
Recognizing whether a medication is a syrup or a suspension can help reinforce proper usage.
- Common Syrups: Many over-the-counter cough and cold remedies are formulated as syrups due to the solubility of their active ingredients and the need for a soothing, palatable vehicle. Examples include products containing guaifenesin (e.g., Robitussin®, Mucinex®) and dextromethorphan (e.g., Delsym®).
- Common Suspensions: A large number of prescription medications are suspensions. This includes the vast majority of liquid antibiotics for children, which are reconstituted from a powder by the pharmacist (e.g., Amoxicillin (Amoxil®), Amoxicillin/Clavulanate (Laamox-CV), Azithromycin (Zithromax®), Cephalexin (Keflex®)). Other examples include antacids, Nystatin antifungal liquid, and sucralfate (Carafate®) for treating ulcers.
FAQ
Why must I shake a suspension but not a syrup?
Because suspensions have solid particles that settle over time, while syrups are true solutions.
Are sugar-free syrups possible?
Yes — sorbitol, xylitol, or artificial sweeteners are used for diabetic patients.
Which is more stable — syrup or suspension?
It depends on the drug. Poorly soluble drugs are often more stable in suspension.
References:
- USP- Pharmacopeia-USP29-NF24 Page 3447
- Indian Pharmacopeia-2014
- What Is an Active Pharmaceutical Ingredient (API)?[verywellHealth]
- Oral Solutions and Suspensions (8/94)[FDA(.gov)]