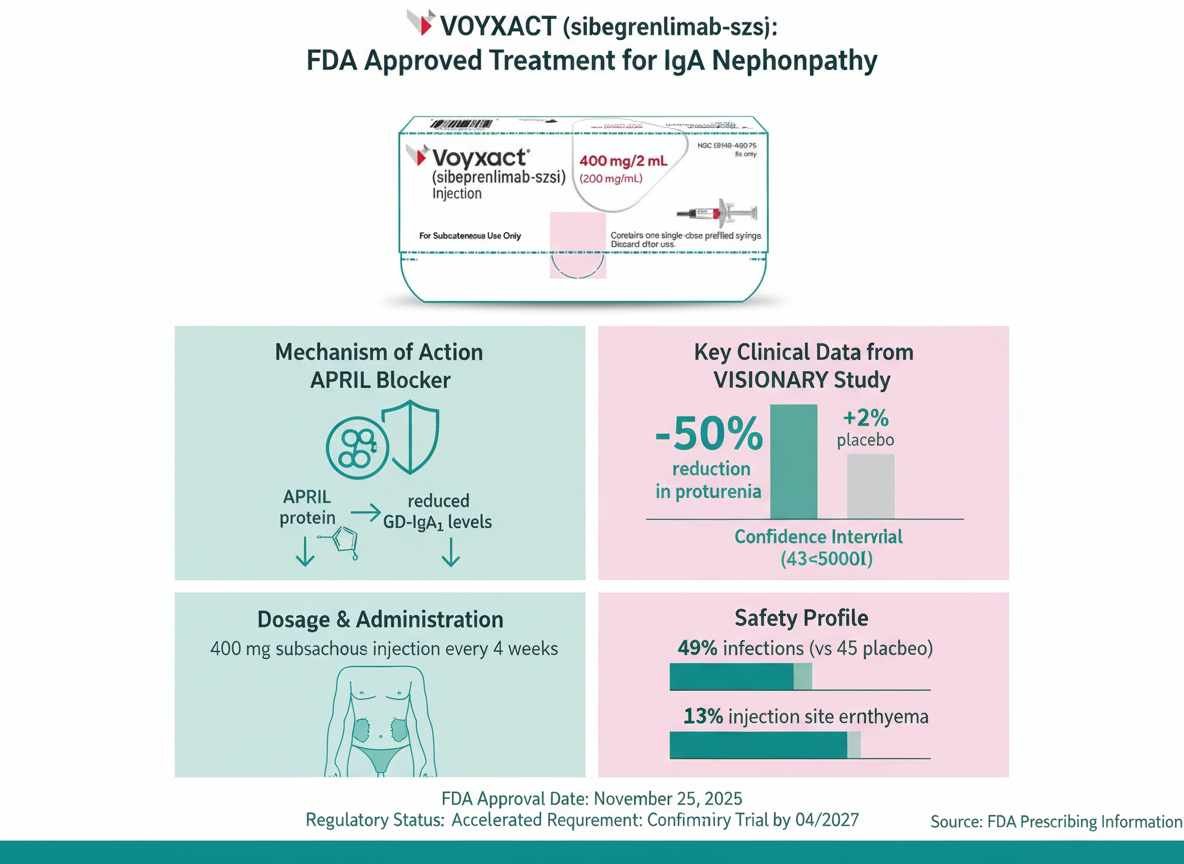
On November 25, 2025, the U.S. Food and Drug Administration (FDA) granted accelerated approval to VOYXACT (sibeprenlimab-szsi), a novel biologic therapy for adults with primary immunoglobulin A nephropathy (IgAN)[3]. Developed by Otsuka Pharmaceutical Company, Ltd., this approval marks a significant advancement in the management of IgAN, a condition that often leads to progressive kidney function decline.
Voyxact is specifically indicated to reduce proteinuria in adults at risk for disease progression. As an accelerated approval, this indication is based on the surrogate endpoint of proteinuria reduction, and continued approval may be contingent upon verification of clinical benefit in a confirmatory trial[2].
Approved Indication & Population
According to the FDA-approved labeling, Voyxact is indicated for:
- Population: Adults with primary immunoglobulin A nephropathy (IgAN).
- Condition: Patients must be at risk for disease progression.
- Goal: To reduce proteinuria[2].
Important Regulatory Context: The approval is under the accelerated pathway (Subpart E). The FDA explicitly states that it has “not been established whether VOYXACT slows kidney function decline over the long-term”[2].
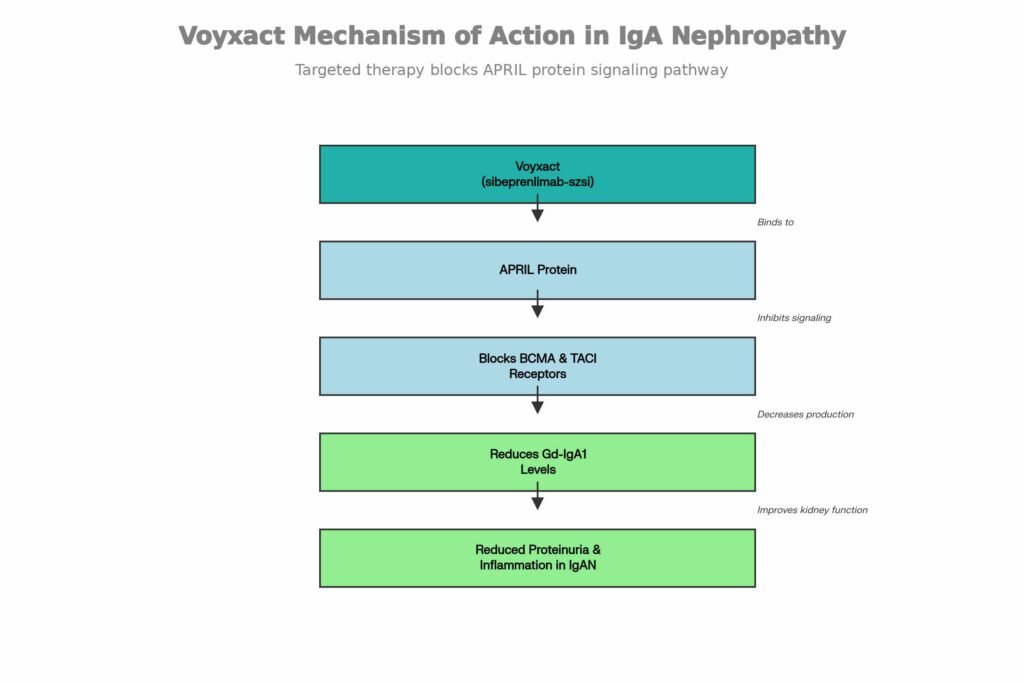
Mechanism of Action
Voyxact operates through a distinct mechanism targeting the pathophysiology of IgAN.
- Drug Class: It is an APRIL (A Proliferation Inducing Ligand) blocker[2].
- Biological Activity: Sibeprenlimab-szsi is a humanized immunoglobulin G2 (IgG2) monoclonal antibody that binds to APRIL with high affinity (dissociation constant of 0.95 pM).
- Pathway Blockade: By binding to APRIL, it blocks signaling at two key receptors:
- BCMA (B cell maturation antigen)
- TACI (transmembrane activator and calcium modulator and cyclophilin ligand interactor)[2].
- Therapeutic Effect: This inhibition results in reduced levels of serum galactose-deficient immunoglobulin A1 (Gd-IgA1), a pathogenic molecule implicated in IgAN[2].
Clinical Trial Design, Endpoints, & Key Efficacy Results
The efficacy of Voyxact was evaluated in the VISIONARY Study (NCT05248646), a randomized, double-blind, placebo-controlled, multicenter global trial[2].
Study Design
- Total Enrollment: 510 patients.
- Interim Analysis: Conducted on the first 320 randomized patients who reached Month 9 (152 Voyxact, 168 Placebo).
- Inclusion Criteria:
- Biopsy-confirmed IgAN.
- eGFR ≥30 mL/min/1.73 m².
- Proteinuria ≥0.75 g/g (uPCR-24h) or ≥1.0 g/day.
- Stable dose of ACE inhibitor (ACEi) and/or Angiotensin Receptor Blocker (ARB), with or without an SGLT2 inhibitor[2].
- Treatment: Randomized 1:1 to Voyxact 400 mg or Placebo subcutaneously every 4 weeks.
Baseline Characteristics
- Median Age: 42 years.
- Mean eGFR: 63 mL/min/1.73 m².
- Mean uPCR-24h: 1.5 g/g.
- Background Therapy: 98% on ACEi/ARB; 40% on SGLT2i[2].
Key Efficacy Results (Month 9)
The primary endpoint was the relative change from baseline in 24-hour urine protein-to-creatinine ratio (uPCR-24h) at Month 9.
| Endpoint | Voyxact Group | Placebo Group | Treatment Difference (95% CI) | p-value |
|---|---|---|---|---|
| % Change in uPCR-24h | -50% | +2% | 51% (43%, 58%) | <0.0001 |
Table Data Source: [Label, Page 8-9][2]
The study demonstrated a statistically significant reduction in proteinuria compared to placebo. Results were consistent across subgroups, including baseline proteinuria levels, eGFR, and SGLT2 inhibitor use[2] .
Safety Profile & Adverse Events
The safety analysis included 259 patients treated with Voyxact and 251 with placebo, with a median exposure of 44 weeks for the Voyxact group[2].
Common Adverse Reactions
Adverse reactions reported in ≥10% of patients treated with Voyxact and at a higher incidence than placebo included:
| Adverse Reaction | Voyxact (n=259) | Placebo (n=251) |
|---|---|---|
| Any Infection | 49% | 45% |
| Injection Site Reactions | 24% | 23% |
| Upper Respiratory Infection | 15% | 14% |
| Injection Site Erythema | 13% | 12% |
Table Data Source: [Label, Page 3][2]
Serious Risks
- Immunosuppression: Voyxact reduces antibody production, potentially increasing the risk of serious infections. In trials, infections occurred in 49% of Voyxact patients vs. 45% for placebo[2].
- Hypersensitivity: Serious hypersensitivity is a contraindication.
- Immunogenicity: 34% of patients developed anti-drug antibodies (ADA). While ADA presence was associated with numerically lower proteinuria reduction (41.6% vs. 52.7%), it did not clinically significantly impact safety[2].
Dosage & Administration
Voyxact is supplied as a 400 mg/2 mL (200 mg/mL) single-dose prefilled syringe[2].
Recommended Dosage
| Parameter | Instruction |
|---|---|
| Dose | 400 mg |
| Frequency | Once every 4 weeks |
| Route | Subcutaneous injection |
| Missed Dose | Administer as soon as possible, then resume dosing every 4 weeks thereafter. |
Table Data Source: [Label, Page 1][2]
Administration Instructions
- Self-Administration: Allowed after proper training.
- Preparation: Allow syringe to reach room temperature (up to 77°F/25°C) for 15 to 30 minutes before injection. Do not use if left at room temperature for 7 days or longer.
- Injection Sites: Front of thigh or abdomen. Caregivers may use the back of the upper arm[2].
- Storage: Store refrigerated at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light[2].
Contraindications, Warnings, & Precautions
Contraindications
- Hypersensitivity: Patients with serious hypersensitivity to sibeprenlimab-szsi or any excipients[2].
Warnings & Precautions
- Immunosuppression and Infections: Assess for active infections before initiation. Monitor for signs of infection during treatment. Consider interrupting therapy if a serious infection develops[2].
- Immunization Risks:
- Live Vaccines: Not recommended within 30 days prior to initiation or during treatment.
- Efficacy: Efficacy of immunizations during treatment has not been established[2].
Pharmacology (PK/PD)
- Bioavailability: Absolute bioavailability is approximately 92% following subcutaneous administration.
- Time to Peak: Median time to reach peak concentration is 8 days.
- Half-Life: Mean terminal half-life is 9.3 days.
- Steady State: Reached by 20 weeks of dosing.
- PD Effect: Serum APRIL levels reduced by >90% by Day 3 and sustained. IgA, IgG, and IgM levels decreased and plateaued by Week 24[2].
Special Populations
Pregnancy
- Risk Summary: No available human data. Monoclonal antibodies can cross the placenta, especially in the second and third trimesters.
- Animal Data: No adverse effects on embryofetal development were observed in monkeys at exposures 10-times the clinical dose.
- Registry: A pregnancy exposure registry is available (1-833-869-9228)[2].
- Postmarketing Requirement: FDA requires a worldwide descriptive study to assess pregnancy outcomes[3].
Pediatric Use
- Status: Safety and effectiveness have not been established.
- Waiver/Deferral: Studies for ages 0 to <2 years were waived (impossible/impracticable). Studies for ages 2 to 17 years are deferred until the product is ready for adults[3].
Lactation
- Data: No data on presence in human milk.
- Postmarketing Requirement: FDA requires a lactation study to measure concentrations in breast milk[3].
Regulatory Timeline & Designations
- Submission Date: March 28, 2025.
- Approval Date: November 25, 2025.
- Applicant: Otsuka Pharmaceutical Company, Ltd.
- Regulatory Pathway: Accelerated Approval under Section 506(c) of the FDCA.
- Confirmatory Trial: FDA requires a randomized, double-blind, placebo-controlled trial to verify clinical benefit, with a final report due by April 2027[3].
FAQs
Is Voyxact a cure for IgA Nephropathy?
No. Voyxact is indicated to reduce proteinuria. It has not been established whether it slows kidney function decline over the long term[2].
Can I take vaccines while on Voyxact?
Live vaccines are not recommended during treatment or within 30 days prior to starting. The efficacy of other immunizations while on Voyxact is unknown[2].
How often do I need to take Voyxact?
The recommended dosage is one 400 mg injection every 4 weeks[2].
Summary
VOYXACT (sibeprenlimab-szsi) represents a breakthrough in IgA nephropathy treatment. This FDA-approved monoclonal antibody blocks APRIL signaling, reducing proteinuria by 50% compared to placebo in the VISIONARY trial. Administered as a 400 mg subcutaneous injection every 4 weeks, Voyxact offers adults with primary IgAN at risk for disease progression a novel therapeutic option. However, accelerated approval means verification of long-term clinical benefit is required. Common adverse events include infections (49%) and injection site reactions (24%). Most effects were mild-to-moderate. Ongoing confirmatory trials will establish whether Voyxact truly slows kidney function decline in this patient population.
Stay Updated on Pharma Approvals & Regulatory Changes
Don’t miss critical FDA decisions that impact patient care.
Follow us for Free Regulatory Alert Newsletter for:
- ✓ Latest FDA drug approvals (summary within 48 hours)
- ✓ Regulatory guidance documents & compliance updates
- ✓ Manufacturing standards & GMP insights
- ✓ Quarterly industry trend analysis
References
- FDA Novel Drug Therapy Approvals for 2025[https://www.fda.gov/drugs/novel-drug-approvals-fda/novel-drug-approvals-2025]
- HIGHLIGHTS OF PRESCRIBING INFORMATION[https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/761434s000lbl.pdf]
- BLA ACCELERATED APPROVAL [https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2025/761434Orig1s000ltr.pdf]