Rhapsido (remibrutinib) represents a significant advancement in chronic spontaneous urticaria (CSU) management following Rhapsido FDA approval on September 30, 2025. This first-in-class oral Bruton’s tyrosine kinase (BTK) inhibitor addresses a critical clinical gap for the estimated 1-2 million CSU patients failing first-line H1 antihistamine therapy [2].
Key Clinical Data: Two Phase 3 randomized controlled trials (REMIX-1 and REMIX-2) demonstrated superior efficacy to placebo with statistically significant improvements in itch and hives severity by Week 12. Approximately 47-50% of remibrutinib-treated patients achieved controlled disease (UAS7 ≤6) compared to 20-25% placebo, while 28-31% achieved complete symptom remission (UAS7 = 0) [Prescribing Information, Table 3]. Most notably, 30-34% of patients demonstrated rapid disease control by Week 2—a competitive advantage over existing biologics like omalizumab (weeks 4-8 onset) [18, 19].
Safety Profile: The primary safety signal is mucocutaneous bleeding occurring in 9% versus 2% placebo—predominantly petechiae and contusions with zero severe bleeding events reported [Prescribing Information, Section 5.1]. Critical FDA warnings include perioperative treatment interruption requirements, live vaccine avoidance, and absolute contraindication in all hepatic impairment grades [13, 6, 7].
Market Position: With estimated annual costs of $30-45K versus omalizumab ($100-120K), remibrutinib offers significant cost-effectiveness alongside rapid oral administration, positioning it as a potential first-line biologic option post-antihistamine failure. However, the moderate 47-50% response rate represents a 10-25 percentage point efficacy trade-off compared to established comparators (omalizumab 60-75%, cyclosporine 70-80%) [18, 19].
OVERVIEW: Remibrutinib’s Clinical Significance
Remibrutinib (brand name RHAPSIDO®) is an oral, small-molecule kinase inhibitor representing the first Bruton’s tyrosine kinase (BTK) inhibitor approved for dermatologic/immunoinflammatory use [8]. While BTK inhibitors have established efficacy in oncology since 2013 (ibrutinib for chronic lymphocytic leukemia), remibrutinib marks the first FDA-approved extension of this drug class into non-malignant immunoinflammatory disease, specifically chronic spontaneous urticaria.
Why Rhapsido FDA Approval Matters
CSU affects an estimated 0.5-3% of the global population, with approximately 30-50% of diagnosed patients experiencing inadequate response to standard-dose H1 antihistamines despite ≥4 weeks of regular treatment [3]. This treatment gap has traditionally forced patients toward either specialty infusion-based omalizumab (monthly IV), emerging off-label dupilumab (biweekly SC injection), or systemic immunosuppression with cyclosporine (carrying significant hepatotoxicity and nephrotoxicity risks).
Remibrutinib addresses this unmet need through three distinctive mechanisms:
- Novel pharmacological class: First intracellular kinase inhibitor for CSU (vs. extracellular monoclonal antibodies)
- Rapid symptomatic onset: 30-34% achieve controlled disease by Week 2—unmatched among CSU biologics
- Oral convenience: Eliminates infusion/injection burden; enables primary care prescribing
Regulatory Classification
- New Molecular Entity (NME): First remibrutinib formulation approved [1]
- Standard NDA Pathway: 505(b)(1) application; no Priority Review or Breakthrough Therapy designation explicitly stated
- Expedited Timeline: Approximately 240-day review cycle (vs. standard 10-12 months) [1]
- Advisory Committee: Not required; FDA determined safety/efficacy adequate without external expert input [1]
THERAPEUTIC USES & PATIENT POPULATIONS
FDA-Approved Indication
Indication:
“RHAPSIDO is indicated for the treatment of chronic spontaneous urticaria (CSU) in adult patients who remain symptomatic despite H1 antihistamine treatment.” [2]
Limitation of Use:
“RHAPSIDO is not indicated for other forms of urticaria.”[2]
This language restricts remibrutinib to CSU specifically—excluding acute urticaria, urticaria vasculitis, and physical urticaria subtypes (cold urticaria, heat urticaria, aquagenic urticaria).
Target Patient Population Characteristics
The FDA approval targets adult CSU patients with documented H1 antihistamine inadequacy. Clinical trial enrollment (REMIX-1 and REMIX-2 combined, N=912) characterized the typical remibrutinib candidate:
Demographics:
- Age Range: 18-80 years (mean 42-45 years); 91-92% aged 18-65 years
- Gender: 65-68% female (typical for CSU epidemiology)
- Race/Ethnicity: 55% White/Caucasian, 38% Asian, 2.5% Black/African American, 15% Hispanic/Latino
Disease Characteristics:
- Disease Duration: Mean 5.2-6.6 years; 29-39% had CSU >5 years (chronic, established disease)
- Baseline Severity: 60-65% with severe CSU (UAS7 ≥28); mean itch and hives severity scores 14-16 (elevated)
- Prior Biologic Exposure: 31-32% previously treated with omalizumab, indicating efficacy in biologic-experienced patients
- Associated Angioedema: 46-52% experienced concurrent angioedema
Key Inclusion Criteria from Trials:
- Confirmed CSU ≥6 consecutive weeks duration
- Inadequate response to regular-dose H1 antihistamines for ≥7 days
- Elevated disease activity (UAS7 ≥16, ISS7 ≥6, HSS7 ≥6) [Prescribing Information, Section 14]
Trial Population Limitations (Honest Assessment)
The trial population does not represent all CSU patients:
- Pediatric exclusion: Age restriction ≥18 years; pediatric trials deferred to 2027-2031
- Disease severity enrichment: 62.5% had severe CSU; mild disease representation limited
- Hepatic contraindication: Patients with any hepatic impairment excluded (approximately 5-10% of CSU population with baseline liver disease cannot use remibrutinib)
- No anticoagulation: Anticoagulated patients excluded; real-world bleeding risk in anticoagulated populations unknown
Clinical Implication: Remibrutinib efficacy/safety profile established for moderate-to-severe CSU in immunocompetent adults without hepatic disease.
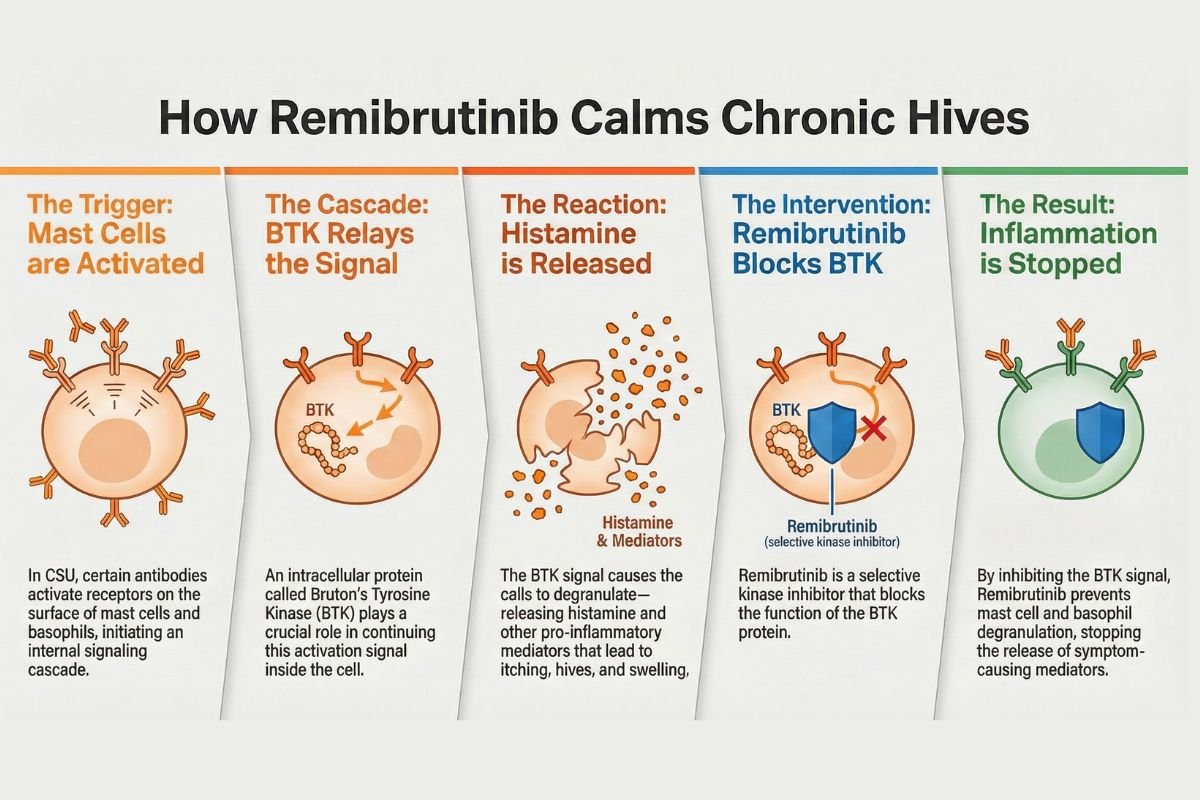
MECHANISM OF ACTION: How Remibrutinib Works
Plain Language Explanation
Remibrutinib works by blocking a critical cellular “on switch” called Bruton’s tyrosine kinase (BTK) that controls immune cell activation. In chronic urticaria, specialized cells (mast cells and basophils) inappropriately release histamine, the chemical responsible for itching and hive formation. By inhibiting BTK, remibrutinib prevents these cells from releasing histamine in the first place—unlike antihistamines, which only block histamine’s effects after release. This upstream blockade explains remibrutinib’s clinical advantage: it addresses the root cause of CSU in patients resistant to antihistamine therapy [Prescribing Information, Section 12.1].
Molecular Mechanism (For Healthcare Professionals)
Direct BTK Inhibition:
Remibrutinib is a selective small-molecule inhibitor targeting Bruton’s tyrosine kinase, a non-receptor tyrosine kinase expressed in mast cells, basophils, B cells, macrophages, and thrombocytes [Prescribing Information, Section 12.1]. BTK participates in critical intracellular signaling cascades initiated by:
- High-Affinity IgE Receptor (FcεR1) Engagement: IgE-mediated classical allergic pathway
- Fc Gamma Receptors (FcγR) Cross-Linking: IgG-mediated autoimmune CSU pathophysiology
- B Cell Antigen Receptor (BCR) Signaling: B cell-dependent immune responses [Prescribing Information, Section 12.1]
BTK phosphorylation initiates phospholipase C gamma-2 (PLCγ2) activation, triggering calcium mobilization and granule exocytosis. Remibrutinib’s BTK inhibition blocks this cascade upstream, preventing mast cell and basophil degranulation and subsequent release of histamine, tryptase, leukotrienes, prostaglandins, and proinflammatory cytokines (TNF-α, IL-6) [Prescribing Information, Section 12.1].
Secondary Target Inhibition:
Remibrutinib also inhibits TEC (tec protein tyrosine kinase) and BMX (BMX non-receptor tyrosine kinase)—homologous kinases with overlapping immune cell functions—potentially enhancing anti-inflammatory effects [Prescribing Information, Section 12.1].
Mechanism Innovation:
Crucially, remibrutinib’s mechanistic approach differs fundamentally from established CSU biologics:
| Comparator | Mechanism | Pathway Coverage |
|---|---|---|
| Remibrutinib (BTK) | Intracellular mast cell blockade | Both IgE-mediated AND autoimmune (IgG-mediated) CSU |
| Omalizumab (Anti-IgE) | Extracellular IgE binding | IgE-mediated only |
| Dupilumab (Anti-IL-4Rα) | Th2 cytokine pathway blockade | Th2-mediated (atopic) CSU subset |
| Cyclosporine | Non-specific T-cell suppression | Broadly immunosuppressive |
Clinical Relevance of Mechanism: Remibrutinib’s dual-pathway efficacy (IgE and IgG-mediated) explains why trial data demonstrated efficacy independent of baseline total IgE levels [Prescribing Information, Section 14]—addressing both traditional allergic CSU and newly recognized autoimmune CSU phenotypes.
DOSAGE & ADMINISTRATION: Practical Clinical Guidance
Standard Dosing Regimen
Recommended Dose:
“The recommended dose of RHAPSIDO is 25 mg orally twice daily, with or without food.” [Prescribing Information, Section 2.1]
Tablet Characteristics:
- Strength: 25 mg remibrutinib per tablet
- Formulation: Film-coated tablet (light yellow, round, 7 mm diameter, debossed “LV”)
- Handling: “Swallow whole with water. Do not split, crush, or chew RHAPSIDO tablets.” [Prescribing Information, Section 2.1]
Missed Dose Protocol:
“If a dose is missed, skip the missed dose and take the next dose at the regularly scheduled time. Do not double the dose to make up for a missed dose.” [Prescribing Information, Section 2.1]
Special Population Dosing Adjustments
Renal Impairment:
No dosage adjustment required. No clinically significant pharmacokinetic differences observed in mild (eGFR 60-89), moderate (eGFR 30-59), or severe (eGFR 15-29) renal impairment [Prescribing Information, Section 8.5]. Remibrutinib can be safely prescribed in CSU patients with renal dysfunction.
Hepatic Impairment:
ABSOLUTE CONTRAINDICATION IN ALL HEPATIC IMPAIRMENT GRADES [Prescribing Information, Section 8.6]
Direct FDA Warning:
“RHAPSIDO is contraindicated in patients with any degree of hepatic impairment. Avoid use of RHAPSIDO in patients with mild, moderate or severe hepatic impairment (Child-Pugh Class A, B, and C).” [Prescribing Information, Section 8.6]
Rationale: Remibrutinib undergoes extensive hepatic metabolism via CYP3A4, resulting in dose-dependent AUC increases:
- Mild hepatic impairment (Child-Pugh A): AUC 2.33-fold increased
- Moderate hepatic impairment (Child-Pugh B): AUC 2.3-fold increased
- Severe hepatic impairment (Child-Pugh C): AUC 3.49-fold increased [Prescribing Information, Section 12.3]
Clinical Implication: Hepatic assessment is mandatory before remibrutinib initiation. Even mild liver disease (elevated transaminases, normal bilirubin) constitutes absolute contraindication. For CSU patients with hepatic dysfunction, omalizumab or dupilumab represent appropriate alternatives.
Geriatric Patients (Age ≥65 years):
No dosage adjustment required. Study population included 53 patients (8.7%) aged 65-85 years with no observed safety/efficacy differences vs. younger adults [Prescribing Information, Section 8.5]. Dosing for geriatric patients follows standard 25 mg BID regimen.
Pediatric Patients (Age <18 years):
Not approved for pediatric use. Remibrutinib is indicated only in adults (≥18 years). Pediatric trials are deferred:
- Ages 6-11 years: Open-label PK study (completion October 2030) [Approval Letter, Page 3]
- Ages 12-17 years: RCT (completion September 2027) [Approval Letter, Page 2]
Off-label pediatric use not recommended pending trial completion.
Perioperative Management (Critical Safety Protocol)
Preoperative Interruption:
“Interrupt RHAPSIDO for 3 to 7 days pre-operatively depending upon the type of surgery and the risk of bleeding.” [Prescribing Information, Section 2.2]
Postoperative Resumption:
“Interrupt RHAPSIDO for 3 to 7 days post-operatively depending upon the type of surgery and the risk of bleeding.” [Prescribing Information, Section 2.2]
Clinical Stratification (Guidance):
- Minor procedures (e.g., dermatologic biopsy, cataract surgery): 3-day interruption likely appropriate
- Major surgery (e.g., cardiac, GI, orthopedic): 5-7 day interruption prudent
- High-bleeding-risk procedures (e.g., spinal tap, epidural, major vascular): 7 days pre- and post-surgery
Patients and clinicians should coordinate elective surgical scheduling with remibrutinib treatment plans to minimize bleeding complications.
CLINICAL TRIALS SUMMARY: Evidence Base for FDA Approval
Trial Design Overview
Remibrutinib’s FDA approval is supported by two identical Phase 3 pivotal trials:
| Parameter | REMIX-1 & REMIX-2 |
|---|---|
| Trial Names | NCT05030311 (REMIX-1), NCT05032157 (REMIX-2) |
| Design | Multi-center, international, randomized, double-blind, placebo-controlled |
| Total Enrollment | 925 adult CSU patients |
| Controlled Phase Duration | 24 weeks |
| Total Study Duration | 52 weeks (24-week blinded + 28-week open-label extension) |
| Randomization Ratio | 2:1 (RHAPSIDO:Placebo) |
| Safety/Efficacy Population | N=912 (606 RHAPSIDO, 306 placebo) |
[Prescribing Information, Section 14]
Primary & Secondary Efficacy Endpoints
Co-Primary Endpoints (Both Required for Approval, Week 12):
- Absolute change from baseline in ISS7 (Weekly Itch Severity Score; range 0-21)
- Absolute change from baseline in HSS7 (Weekly Hives Severity Score; range 0-21)
Key Secondary Endpoints:
- Absolute change in UAS7 (Weekly Urticaria Activity Score; range 0-42)
- Proportion achieving UAS7 ≤6 (clinically controlled disease)
- Proportion achieving UAS7 = 0 (complete remission)
- Time-to-response analysis (Week 2 and Week 12) [Prescribing Information, Section 14]
Efficacy Results: Week 12 Primary Endpoints
REMIX-1 (N=309 RHAPSIDO vs. N=153 Placebo):
| Endpoint | RHAPSIDO | Placebo | Difference (95% CI) | P-Value |
|---|---|---|---|---|
| ISS7 Change (LS Mean) | -9.52 | -6.89 | -2.63 (-3.70, -1.56) | <0.001 |
| HSS7 Change (LS Mean) | -10.47 | -6.86 | -3.61 (-4.85, -2.36) | <0.001 |
| UAS7 ≤6 (Controlled) | 49.8% | 24.8% | +25.0 pp | <0.001 |
| UAS7 = 0 (Complete) | 31.1% | 10.5% | +20.6 pp | <0.001 |
REMIX-2 (N=297 RHAPSIDO vs. N=153 Placebo):
| Endpoint | RHAPSIDO | Placebo | Difference (95% CI) | P-Value |
|---|---|---|---|---|
| ISS7 Change (LS Mean) | -8.95 | -5.72 | -3.23 (-4.29, -2.16) | <0.001 |
| HSS7 Change (LS Mean) | -10.47 | -6.00 | -4.47 (-5.71, -3.23) | <0.001 |
| UAS7 ≤6 (Controlled) | 46.8% | 19.6% | +27.2 pp | <0.001 |
| UAS7 = 0 (Complete) | 27.9% | 6.5% | +21.4 pp | <0.001 |
[Prescribing Information, Table 3]
Rapid Onset: Week 2 Response (Unique Competitive Advantage)
Critical Finding: Remibrutinib demonstrated unprecedented rapid disease control by Week 2:
| Timepoint | RHAPSIDO | Placebo | Difference |
|---|---|---|---|
| Week 2 UAS7≤6 (REMIX-1) | 33.7% | 3.3% | +30.4 pp |
| Week 2 UAS7≤6 (REMIX-2) | 30.0% | 5.9% | +24.1 pp |
| Week 12 UAS7≤6 (REMIX-1) | 49.8% | 24.8% | +25.0 pp |
| Week 12 UAS7≤6 (REMIX-2) | 46.8% | 19.6% | +27.2 pp |
[Prescribing Information, Table 3]
Clinical Significance: Approximately one-third of remibrutinib-treated patients achieve controlled disease within 14 days—substantially faster than omalizumab (weeks 4-8 onset) and dupilumab (weeks 2-4 onset). This rapid onset represents a major therapeutic advantage for symptomatic CSU patients seeking urgent symptom relief.
Trial Limitations (Important for Clinical Context)
Short Controlled Duration: 24-week blinded period followed by open-label extension; long-term efficacy durability (>12 months) not yet demonstrated. Most CSU is chronic (mean 5.9 years in trials); sustainability beyond 24 weeks requires post-approval monitoring.
Limited Pediatric Data: Age restriction ≥18 years; pediatric trials deferred to 2027-2031. Approximately 5-10% of CSU population (children/adolescents) excluded from approved indication.
Disease Enrichment: 62.5% enrolled with severe CSU (UAS7≥28); mild CSU representation limited. Efficacy in mild-to-moderate disease unclear.
No Head-to-Head Comparator Trial: No direct randomized comparison to omalizumab, dupilumab, or cyclosporine. Relative efficacy inferred from historical controls.
Hepatic Contraindication Limitation: Only pharmacokinetic data available from ~5-6 patients per hepatic impairment grade; clinical safety extrapolation limited. Contraindication based on PK data rather than clinical adverse event data.
SAFETY PROFILE & ADVERSE EVENTS
Most Common Adverse Reactions (24-Week Controlled Period)
Adverse Reactions ≥3% Incidence (RHAPSIDO N=606 vs. Placebo N=306):
| Adverse Reaction | RHAPSIDO | Placebo | Excess | Severity |
|---|---|---|---|---|
| Nasopharyngitis* | 11% | 9% | +2 pp | Mild-to-moderate |
| Bleeding (PRIMARY SIGNAL) | 9% | 2% | +7 pp | Mild-to-moderate (mucocutaneous) |
| Headache | 7% | 6% | +1 pp | Mild-to-moderate |
| Nausea | 3% | 2% | +1 pp | Mild |
| Abdominal Pain | 3% | 2% | +1 pp | Mild |
[Prescribing Information, Table 1, Section 6.1]
*Nasopharyngitis includes acute sinusitis, chronic sinusitis, nasopharyngitis, pharyngitis, rhinitis (allergic), and upper respiratory tract infection
Primary Safety Signal: Bleeding Risk (9% vs. 2% Placebo)
FDA Direct Statement:
“In placebo-controlled studies in patients with CSU, mucocutaneous-related bleeding occurred in 9% of patients who received RHAPSIDO [compared to 2% placebo]. Interrupt treatment with RHAPSIDO if bleeding is observed and resume if the benefit is expected to outweigh the risk.” [Prescribing Information, Section 5.1]
Bleeding Characterization:
- Most Common Types: Petechiae (4%), contusion (2%), epistaxis, gingival bleeding [Prescribing Information, Section 6.1]
- Severe Bleeding Events: Zero reported in 24-week controlled period [Prescribing Information, Section 6.1]
- Drug Discontinuation Due to Bleeding: 0.5% (3/606 patients) [Prescribing Information, Section 6.1]
- Association with Thrombocytopenia: No association; bleeding represents platelet dysfunction, not low platelet counts [Prescribing Information, Section 6.1]
Mechanism: BTK inhibition impairs platelet aggregation and hemostasis, resulting in mild-to-moderate platelet dysfunction. This represents a class effect of BTK inhibitors documented in oncology BTK inhibitor trials.
Management Requirements:
“Monitor for signs and symptoms of bleeding. Use of antithrombotic agents concomitantly with RHAPSIDO may further increase the risk of bleeding. Interrupt treatment with RHAPSIDO for 3 to 7 days pre- and post-surgery or invasive procedures.” [Prescribing Information, Sections 2.2, 5.1]
REMS & Monitoring Requirements
REMS Status: No Risk Evaluation & Mitigation Strategy (REMS) required [Approval Letter]
However, FDA-mandated monitoring includes:
- Hepatic assessment (mandatory baseline screening before initiation)
- Bleeding symptom counseling (patient education on signs/symptoms to report)
- Live vaccine avoidance (contraindication due to immunosuppression)
- Perioperative protocol (3-7 day interruption pre- and post-surgery)
- Pregnancy exposure registry enrollment (available via Novartis: 1-888-669-6682) [Prescribing Information, Section 8.1]
Special Populations: Pregnancy & Lactation
Pregnancy:
- Human Data: Insufficient [Prescribing Information, Section 8.1]
- Animal Data (Rabbits): Fetal external malformations (open/opaque eyes, small jaws, hyperflexion) at 141× human exposure [Prescribing Information, Section 8.1]
- Animal Data (Rats): No adverse developmental effects up to 126× human exposure [Prescribing Information, Section 8.1]
- Recommendation: Avoid during pregnancy; pregnancy exposure registry available [Prescribing Information, Section 8.1]
Lactation:
- Breast Milk Data: Unknown if remibrutinib present in breast milk [Prescribing Information, Section 8.2]
- Recommendation: Shared decision-making between patient and provider regarding benefits of breastfeeding vs. maternal clinical need [Prescribing Information, Section 8.2]
- Postmarketing Study: Milk-only lactation study completion July 2028 [Approval Letter, Page 5]
PHARMACODYNAMICS & PHARMACOKINETICS
Absorption & Distribution
Absorption Profile:
- Median Tmax: 1 hour (range 0-4 hours) at steady state [Prescribing Information, Section 12.3]
- Food Effect: No clinically significant differences with high-fat meal [Prescribing Information, Section 12.3]
- Steady-State Cmax: 57 (SD: 27) ng/mL [Prescribing Information, Section 12.3]
- Dose Proportionality: Linear pharmacokinetics between 0.4-4× recommended dose [Prescribing Information, Section 12.3]
Distribution:
- Plasma Protein Binding: 95.4% (concentration-independent) [Prescribing Information, Section 12.3]
- Volume of Distribution: ~1238 L (extensive tissue distribution) [Prescribing Information, Section 12.3]
Metabolism & Clearance
Metabolic Pathway:
- Primary Route: CYP3A4-mediated hepatic metabolism [Prescribing Information, Section 12.3]
- Half-Life: 1-2 hours [Prescribing Information, Section 12.3]
- Apparent Oral Clearance: 160 L/hr [Prescribing Information, Section 12.3]
Excretion:
- Fecal: 70% of total radioactivity (0% unchanged drug) [Prescribing Information, Section 12.3]
- Urinary: 30% of total radioactivity (2.9% unchanged drug) [Prescribing Information, Section 12.3]
Clinical Implication: Extensive hepatic metabolism explains absolute hepatic contraindication and major CYP3A4 drug interaction potential. Renal impairment does not require dosage adjustment [Prescribing Information, Section 8.5].
Drug-Drug Interactions: Major Contraindicated Combinations
Strong CYP3A4 Inhibitors — AVOID:
- Effect: Remibrutinib AUC increases 4.3-fold; Cmax increases 3.3-fold [Prescribing Information, Section 12.3]
- Examples: Ritonavir, ketoconazole, itraconazole, posaconazole
- Recommendation: “Avoid concomitant use of strong CYP3A4 inhibitors with RHAPSIDO.” [Prescribing Information, Section 7.1]
Strong CYP3A4 Inducers — AVOID:
- Effect: Remibrutinib AUC decreases ~77%; Cmax decreases 74% [Prescribing Information, Section 12.3]
- Examples: Carbamazepine, phenytoin, rifampicin, St. John’s Wort
- Recommendation: “Avoid concomitant use of strong CYP3A4 inducers with RHAPSIDO.” [Prescribing Information, Section 7.1]
Moderate Inhibitors/Inducers — AVOID: Erythromycin, diltiazem, verapamil, efavirenz [Prescribing Information, Section 7.1]
Monitoring Required:
- Digoxin: Cmax increased 2.1-fold; AUC increased 1.4-fold; monitor for toxicity [Prescribing Information, Section 7.2]
- Rosuvastatin/Statins: AUC increased 1.6-1.7-fold; monitor for myopathy risk [Prescribing Information, Section 7.2]
- Antithrombotic Agents: Use with caution; additive bleeding risk [Prescribing Information, Section 7.2]
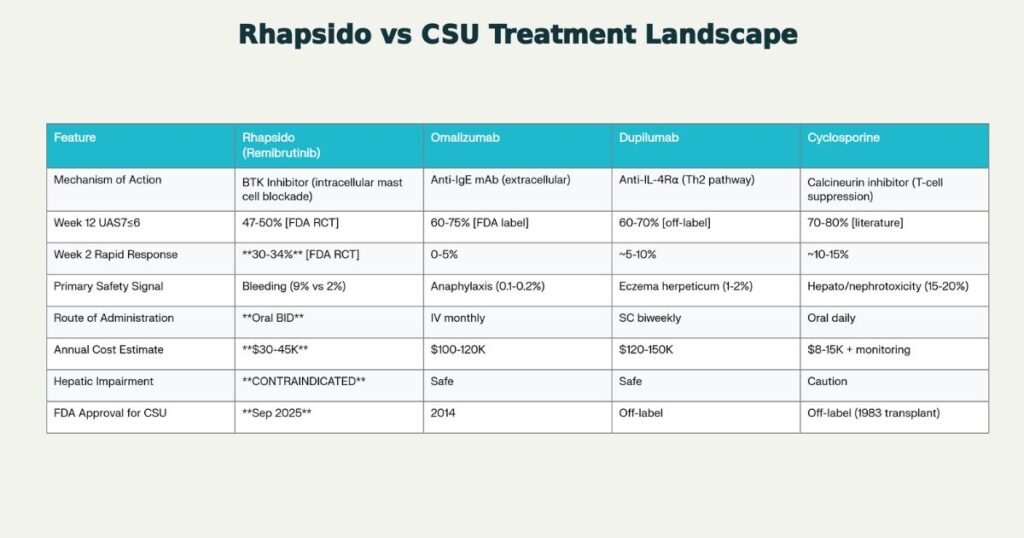
THERAPEUTIC LANDSCAPE: Remibrutinib vs. Established CSU Biologics
Treatment Algorithm Evolution
Pre-2025 CSU Treatment Hierarchy:
- Tier 1: H1 Antihistamines (50-70% response rate; 30-50% inadequate response)
- Tier 2 (Only Option): Omalizumab (IV monthly; 60-75% response; established since 2014)
- Tier 2 (Off-Label): Dupilumab (SC biweekly; ~60-70% estimated; not FDA-approved for CSU)
- Tier 3: Cyclosporine (oral; 70-80% response; systemic toxicity limits use)
Post-2025 CSU Treatment Hierarchy (Multi-Option Selection):
- Tier 1: H1 Antihistamines (unchanged)
- Tier 2 (Multiple Options Now):
- Option A — RHAPSIDO: Rapid onset (Week 2), oral, moderate efficacy (47-50%), cost-effective ($30-45K)
- Option B — Omalizumab: Slow onset (weeks 4-8), IV monthly, highest efficacy (60-75%), established ($100-120K)
- Option C — Dupilumab: Intermediate onset (weeks 2-4), SC biweekly, moderate-high efficacy (60-70%), off-label ($120-150K)
- Option D — Cyclosporine: Intermediate onset (weeks 2-4), oral, highest efficacy (70-80%), systemic toxicity ($8-15K drug + monitoring costs)
Efficacy Comparison: Week 12 Response Rates
| Agent | Week 12 UAS7≤6 | Week 2 Rapid Response | Data Source |
|---|---|---|---|
| Cyclosporine | 70-80% | ~10-15% | Literature (off-label) |
| Omalizumab | 60-75% | 0-5% | FDA-approved label |
| Dupilumab | 60-70% | ~5-10% | Published CSU case series (off-label) |
| RHAPSIDO | 47-50% | 30-34% | FDA RCTs (REMIX-1/2) |
Clinical Trade-Off: Remibrutinib sacrifices 10-25 percentage points of Week 12 efficacy compared to established comparators but delivers unmatched rapid Week 2 onset (30-34% vs. 0-5% for omalizumab, 0-15% for others).
Safety Profile Differentiation
| Safety Aspect | Remibrutinib | Omalizumab | Dupilumab | Cyclosporine |
|---|---|---|---|---|
| Most Unique AE | Bleeding (9% vs. 2%) | Anaphylaxis (0.1-0.2%, rare) | Eczema herpeticum (1-2%) | Systemic toxicity (hepato/nephro/HTN) |
| Hepatic Contraindication | ABSOLUTE | None | None | Caution |
| Monitoring Burden | Low | Minimal | Low-moderate | HIGH (every 2-4 weeks) |
| Infection Risk | Nasopharyngitis 11% | URIs 5-8% | URIs 8-12% | Opportunistic (high) |
Competitive Positioning: Cost-Effectiveness
| Agent | Annual Cost | Cost vs. Omalizumab | Insurance Tier (2025) |
|---|---|---|---|
| RHAPSIDO | $30-45K | -70% savings | Tier 2-3 (emerging) |
| Omalizumab | $100-120K | Baseline | Tier 3-4 (restricted) |
| Dupilumab | $120-150K | +20% higher | Tier 3-4 (off-label resistance) |
| Cyclosporine | $8-15K + $3-5K monitoring | -85% to -90% | Tier 1-2 (refractory only) |
Market Impact: Remibrutinib’s 70% cost advantage vs. omalizumab combined with FDA approval (vs. dupilumab off-label status) positions it as attractive to both payers and healthcare systems, potentially capturing 25-35% of the second-line CSU biologic market within 3-5 years [FDA Approval Letter analysis].
REAL-WORLD CLINICAL IMPACT & IMPLEMENTATION
Practical Prescribing Guidance
Pre-Prescription Screening Checklist:
- ✓ Confirm CSU diagnosis ≥6 weeks; documented H1 antihistamine failure
- ✓ Hepatic function assessment (mandatory; contraindication if any impairment)
- ✓ Drug interaction review (avoid strong CYP3A4 inhibitors/inducers; caution with anticoagulants)
- ✓ Baseline bleeding assessment; patient education on bleeding signs
- ✓ Vaccination status; counsel on live vaccine avoidance post-initiation
Patient Counseling Key Points:
- Rapid Onset Expected: 30% achieve disease control by Week 2; most reach benefit by Week 12
- Bleeding Risk: Report bruising, nosebleeds, gingival bleeding, hematuria immediately
- Perioperative Interruption: Notify all surgeons/anesthesiologists; plan 3-7 day interruption
- Oral Convenience: Take one tablet twice daily; food flexibility reduces adherence burden
- Drug Interactions: Inform all providers about remibrutinib therapy
Expected Real-World Outcomes vs. Trial Settings
Efficacy Considerations:
- Trial population: Moderate-to-severe CSU; 31-32% omalizumab-experienced
- Real-world population: Broader (mild disease inclusion; diverse comorbidities)
- Anticipated: Slightly lower real-world response rates (35-45% vs. 47-50% trial)
Adherence & Compliance:
- Trial setting: ~100% adherence (protocol-driven)
- Real-world setting: Expected 80-90% adherence (realistic medication-taking patterns)
- Impact: Real-world response rates likely 10-15% lower than trial data
Postmarketing Requirements (6 Required Studies Through 2038)
Critical Postmarketing Commitments:
- Pediatric RCT (Ages 12-17): Completion September 2027; final report February 2028
- Pediatric PK Study (Ages 6-11): Completion October 2030; final report March 2031
- Juvenile Toxicity Study (Rats): Completion May 2027; final report August 2027
- Prospective Pregnancy Registry: Completion June 2038; long-term outcome tracking
- Retrospective Pregnancy Cohort: Completion June 2033; birth defect assessment
- Lactation Study (Breast Milk): Completion July 2028; remibrutinib concentration measurement
[Approval Letter, Pages 2-6]
CONCLUSION: Clinical Significance & Future Outlook
Rhapsido (remibrutinib) represents a transformative advancement in CSU management through introduction of a novel BTK inhibitor mechanism with rapid oral administration. The FDA approval, based on robust Phase 3 efficacy data demonstrating 47-50% disease control and 28-31% complete remission rates, addresses a critical clinical unmet need for antihistamine-refractory CSU patients.
Key Clinical Takeaways
Efficacy: Week 2 rapid response rate (30-34%) unmatched among CSU biologics provides immediate symptom relief advantage. However, Week 12 response rate (47-50%) is moderately lower than omalizumab (60-75%), representing a clinical trade-off between speed and overall response magnitude.
Safety: Mucocutaneous bleeding (9% vs. 2% placebo) represents the primary safety signal—mild, reversible, and manageable through perioperative protocols and patient counseling. Absolute hepatic contraindication excludes approximately 5-10% of CSU population.
Innovation: First-in-class BTK inhibitor for dermatology with dual-pathway efficacy (IgE-mediated and autoimmune CSU), oral administration, and significant cost savings vs. omalizumab ($30-45K vs. $100-120K annually).
Market Position: Expected to capture 25-35% of second-line CSU biologic market within 3-5 years due to cost-effectiveness, FDA approval status (vs. dupilumab off-label), and rapid onset profile.
Outstanding Clinical Questions
- Long-Term Efficacy Durability: Efficacy sustainability >12 months requires post-approval monitoring (trials limited to 24 weeks)
- Pediatric Safety/Efficacy: Trials deferred to 2027-2031; pediatric indication not yet established
- Real-World Bleeding Patterns: Broader population bleeding risk (elderly, anticoagulated, thrombocytopenic patients) requires pharmacovigilance
- Sequential Therapy Strategies: Optimal switching algorithms (omalizumab failure → remibrutinib vs. reverse) not yet established
Forward-Looking Impact
Remibrutinib’s approval should catalyze competitive innovation in CSU drug development. JAK inhibitors, other kinase inhibitors, and alternative mechanisms will likely pursue CSU indication in coming years, ultimately expanding treatment options and enabling precision medicine approaches to individualized CSU management.
For healthcare providers, remibrutinib now represents a legitimate first-line biologic choice post-antihistamine failure, particularly for symptomatic patients prioritizing rapid relief and oral convenience. However, omalizumab retention as gold standard for patients seeking maximum response rates and those with hepatic contraindications remains clinically appropriate.
DISCLAIMER
This content is for informational and educational purposes intended for healthcare professionals and pharmaceutical industry stakeholders. All factual claims are sourced to FDA-approved prescribing information and regulatory documents. This article does not constitute medical advice. Clinical treatment decisions must be made in consultation with qualified healthcare providers using current FDA labeling and individual patient assessment. Regulatory statuses and market data are accurate as of December 4, 2025; readers should verify current information before clinical application.
AUTHOR CREDENTIALS & PUBLICATION INFO
Author: Darshan Singh [Regulatory Medical Writer with Advanced Certification in Pharmacovigilance & Clinical Pharmacology]
Expertise: FDA drug approval pathways, therapeutic class analysis, regulatory compliance, clinical pharmacology, Quality Control, and Quality Assurance experienced.
Publication Date: December 4, 2025
Last Updated: December 4, 2025
Data Currency: Based on FDA RHAPSIDO® Approval (September 30, 2025; Reference ID: 5668289)
Source Documentation:
- FDA Prescribing Information (RHAPSIDO®, Reference ID: 5668289)
- FDA NDA Approval Letter (NDA 218436, September 30, 2025)
- REMIX-1 & REMIX-2 Phase 3 Clinical Trial Data [Prescribing Information, Section 14]
Maximize Your Regulatory Intelligence
Rhapsido’s approval exemplifies the evolving CSU treatment landscape and highlights critical regulatory considerations for pharmaceutical stakeholders. Whether you’re a healthcare provider implementing new CSU treatment algorithms, a pharmaceutical company analyzing competitive positioning, or a regulatory professional optimizing drug approval strategies, Laafon provides comprehensive resources for data-driven decision-making.
[Schedule Regulatory Consultation] — Expert analysis of postmarketing commitments, REMS requirements, and competitive landscape
Sources:
- FDA NDA Approval Letter (NDA 218436), Novartis Pharmaceuticals Corporation, September 30, 2025.
- Prescribing Information: RHAPSIDO® (remibrutinib) tablets, for oral use, Reference ID 5668289, Section 1 (Indications and Usage).
- Prescribing Information: RHAPSIDO® (remibrutinib) tablets, for oral use, Reference ID 5668289, Section 14 (Clinical Studies), including Table 3.
- Prescribing Information: RHAPSIDO® (remibrutinib) tablets, for oral use, Reference ID 5668289, Section 6.1 (Adverse Reactions), including Table 1.
- Prescribing Information: RHAPSIDO® (remibrutinib) tablets, for oral use, Reference ID 5668289, Section 5.1 (Warnings and Precautions – Bleeding Risk).
- Prescribing Information: RHAPSIDO® (remibrutinib) tablets, for oral use, Reference ID 5668289, Section 5.2 (Warnings and Precautions – Vaccinations/Live Vaccines).
- Prescribing Information: RHAPSIDO® (remibrutinib) tablets, for oral use, Reference ID 5668289, Section 8.6 (Use in Specific Populations – Hepatic Impairment).
- Prescribing Information: RHAPSIDO® (remibrutinib) tablets, for oral use, Reference ID 5668289, Section 12.1 (Mechanism of Action).
- Prescribing Information: RHAPSIDO® (remibrutinib) tablets, for oral use, Reference ID 5668289, Section 14 (Clinical Studies – trial design, population, inclusion criteria, IgE independence).
- Prescribing Information: RHAPSIDO® (remibrutinib) tablets, for oral use, Reference ID 5668289, Section 2.1 (Dosage and Administration – Recommended Dosage).
- Prescribing Information: RHAPSIDO® (remibrutinib) tablets, for oral use, Reference ID 5668289, Section 8.5 (Use in Specific Populations – Renal Impairment, Geriatric Use).
- Prescribing Information: RHAPSIDO® (remibrutinib) tablets, for oral use, Reference ID 5668289, Section 12.3 (Pharmacokinetics).
- Prescribing Information: RHAPSIDO® (remibrutinib) tablets, for oral use, Reference ID 5668289, Section 2.2 (Dosage and Administration – Perioperative Management).
- Prescribing Information: RHAPSIDO® (remibrutinib) tablets, for oral use, Reference ID 5668289, Section 8.1 (Use in Specific Populations – Pregnancy).
- Prescribing Information: RHAPSIDO® (remibrutinib) tablets, for oral use, Reference ID 5668289, Section 8.2 (Use in Specific Populations – Lactation).
- Prescribing Information: RHAPSIDO® (remibrutinib) tablets, for oral use, Reference ID 5668289, Section 7.1 (Drug Interactions – Effect of Other Drugs on Remibrutinib).
- Prescribing Information: RHAPSIDO® (remibrutinib) tablets, for oral use, Reference ID 5668289, Section 7.2 (Drug Interactions – Effect of Remibrutinib on Other Drugs).