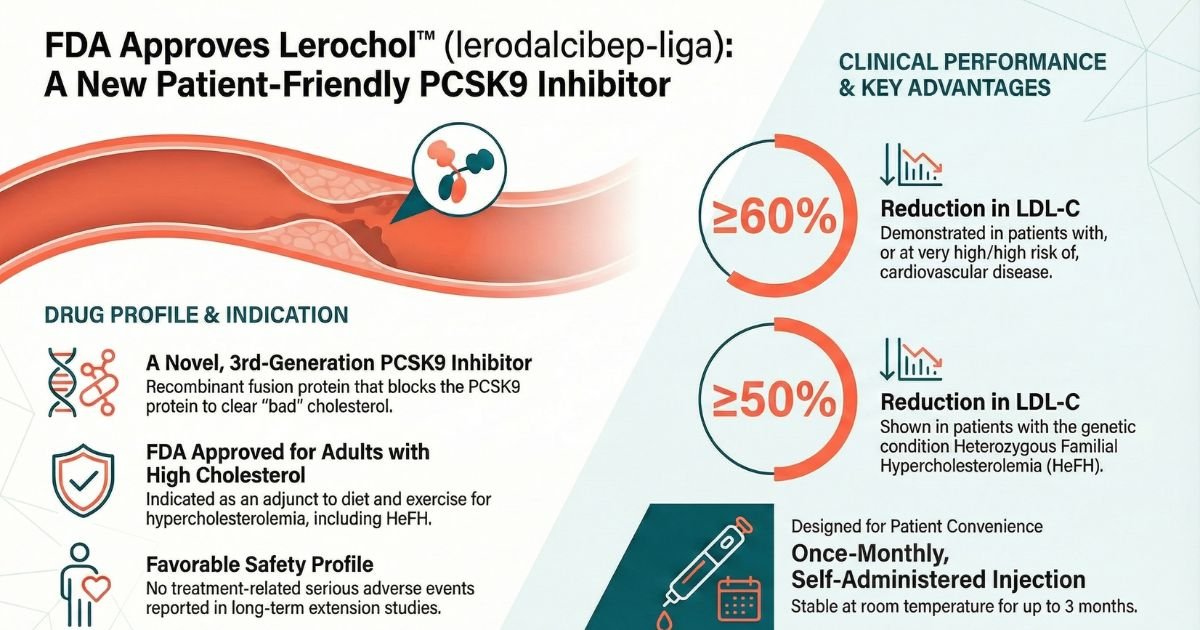
On December 12, 2025, the U.S. Food and Drug Administration (FDA) approved Lerochol (lerodalcibep-liga), a novel third-generation proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor developed by LIB Therapeutics, Inc. Lerochol is approved as an adjunct to diet and exercise to reduce low-density lipoprotein cholesterol (LDL-C) in adults with primary hypercholesterolemia, including heterozygous familial hypercholesterolemia (HeFH)[1, 2].
This approval introduces a distinct therapeutic option into the lipid-management landscape: a recombinant fusion protein designed for once-monthly dosing with extended room-temperature stability. By addressing logistical barriers associated with earlier biologic therapies—such as cold-chain requirements and injection volume—Lerochol aims to support adherence in high-risk populations who have not achieved LDL-C goals on maximally tolerated statin therapy[1].

Quick Reference Table: Lerochol (lerodalcibep-liga)
| Field | Value | Source |
| Generic Name | lerodalcibep-liga | [FDA Approval Letter][2] |
| Brand Name | Lerochol | [FDA Approval Letter][2] |
| Indication | Adjunct to diet and exercise to reduce LDL-C in adults with hypercholesterolemia, including HeFH. | [Prescription Information Section 1][1] |
| Mechanism | Fusion protein binding PCSK9, inhibiting LDLR degradation and increasing hepatic clearance of LDL-C from the bloodstream. | [Prescription Information Section 12.1][1] |
| Primary Endpoint Result | -55% (Trial 1) and -50% (Trial 2) placebo-adjusted LDL-C reduction at Week 52 ($p<0.0001$). | [Prescription Information Section 14][1] |
| Top 3 Side Effects | 1. Nasopharyngitis (15%) 2. Injection site reactions (12%) 3. Diarrhea (3% in HeFH population) | [Prescription Information Section 6.1][1] |
| BLACK BOX WARNING | NO | |
| Approval Date | December 12, 2025 | [FDA Approval Letter][2] |
| Trial N | N=2,017 (Total patients in pivotal Trials 1, 2, and 3) | [Prescription Information Section 14][1] |
Disease and Treatment Landscape
Cardiovascular disease (CVD) remains the leading cause of death globally, with elevated LDL-C recognized as a primary, modifiable risk factor. Despite the widespread availability of statins, a significant proportion of patients fail to reach guideline-recommended LDL-C targets due to intolerance, adherence issues, or the severity of their baseline hypercholesterolemia.
This gap is particularly pronounced in patients with Heterozygous Familial Hypercholesterolemia (HeFH), a genetic condition affecting approximately 1 in 200 individuals worldwide7. HeFH is characterized by severely elevated cholesterol levels from birth, requiring lifelong, aggressive lipid-lowering therapy to prevent premature cardiovascular events[1].
Current standards of care typically involve high-intensity statins, often combined with ezetimibe. For patients requiring further reduction, PCSK9 inhibitors (monoclonal antibodies like evolocumab and alirocumab) and small interfering RNAs (inclisiran) have become established third-line options[1]. However, barriers such as frequent dosing schedules, large injection volumes, and strict refrigeration requirements have historically limited the uptake and long-term adherence to these injectable biologics. Lerochol enters this landscape as a therapeutic specifically engineered to mitigate these practical challenges while maintaining potent efficacy[1].
Mechanism of Action: Lerochol
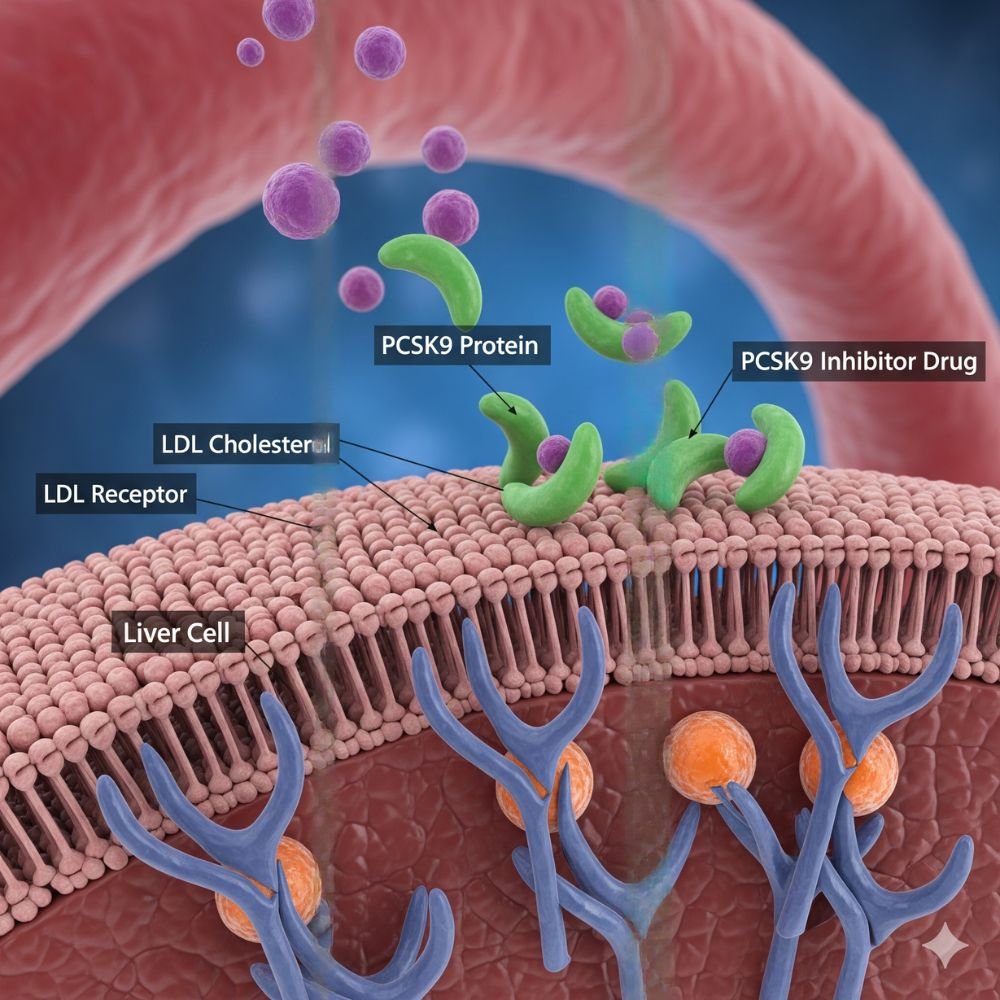
Lerochol (lerodalcibep-liga) functions as a PCSK9 inhibitor but utilizes a distinct molecular architecture compared to first-generation monoclonal antibodies. It is a recombinant fusion protein composed of two functional domains[1]:
- Adnectin Domain: An 11-kDa polypeptide derived from the human tenth fibronectin type III domain. This component is engineered for high-affinity, subnanomolar binding to the PCSK9 protein[1].
- Human Serum Albumin (HSA): The adnectin is fused to HSA, an abundant blood protein. This fusion significantly extends the molecule’s plasma half-life, enabling a once-monthly dosing interval[1].
Physiological Effect: PCSK9 naturally binds to Low-Density Lipoprotein Receptors (LDLR) on the surface of hepatocytes, promoting their degradation within the liver. By binding to circulating PCSK9 with picomolar affinity, Lerochol blocks the interaction between PCSK9 and the LDLR. This inhibition prevents receptor degradation, increasing the density of LDLRs available on the liver surface to clear LDL-C from the bloodstream[1].
Pharmacodynamic data indicate that Lerochol achieves greater than 90% suppression of free PCSK9 within 24 hours of administration, an effect that is maintained throughout the monthly dosing interval[1].
Layperson Analogy: Think of Lerochol as a “bodyguard” for the liver’s cholesterol collectors. It captures the protein that normally destroys these collectors, allowing them to safely remain on the job and continue clearing bad cholesterol from the blood.
Pivotal Clinical Evidence
The FDA approval was supported by the global Phase 3 LIBerate Clinical Trial Program, which enrolled over 2,900 patients across diverse high-risk populations. The program demonstrated consistent, potent LDL-C reduction in patients on maximally tolerated statin therapy[1, 2].
Trial 1 (NCT04797247) & Trial 2 (NCT04806893): Adults with ASCVD or High Risk
These were 52-week, randomized, double-blind, placebo-controlled trials enrolling 1,844 adults with established atherosclerotic cardiovascular disease (ASCVD) or at increased risk for ASCVD events. Patients were randomized 2:1 to receive Lerochol 300 mg or placebo monthly.[6]
- Primary Endpoint (Trial 1): The placebo-adjusted percent change in LDL-C from baseline to Week 52 was -55% (95% CI: -59.2%, -50.8%; p<0.0001)[1, 2].
- Primary Endpoint (Trial 2): The placebo-adjusted percent change in LDL-C from baseline to Week 52 was -50% (95% CI: -54.2%, -45.2%; p<0.0001).
- Secondary Endpoints (Trial 1): Lerochol significantly reduced non-HDL-C (-45% vs placebo), ApoB (-40% vs placebo), and total cholesterol (-31% vs placebo)[1, 2][6].
Trial 3 (LIBerate-HeFH, NCT04797104): Heterozygous Familial Hypercholesterolemia
This 24-week trial enrolled 478 patients with HeFH. The population had a high baseline LDL-C (mean 150 mg/dL) despite 89% being on statin therapy (67% on high-intensity doses).
- Primary Endpoint: The difference in mean percentage change in LDL-C from baseline to Week 24 was -59% (95% CI: -65.7%, -51.7%; p<0.0001)[7].
- Target Achievement: Nearly 70% of treated patients achieved both a ≥50% reduction in LDL-C and met recommended LDL-C targets[7].
- Atherogenic Markers: Significant reductions were observed in Apolipoprotein B (mean -45.6%) and Lipoprotein(a) [Lp(a)] (median -24.4%) compared to placebo[7].
Limitations and Data Gaps
While efficacy is robust, the current approval is based on surrogate endpoints (LDL-C reduction) rather than cardiovascular outcomes (CVOT) data, which is standard for this class at initial approval32. Long-term safety data beyond 72 weeks is currently limited to open-label extensions33. Pediatric efficacy has not yet been established; a study in ages 10–17 (NCT04034485) did not demonstrate effectiveness in homozygous FH, and studies for HeFH are deferred34343434.
Safety and Risk Management
Lerochol displays a safety profile consistent with the PCSK9 inhibitor class. There is no Black Box Warning in the prescribing information.
Adverse Reactions
In the pooled 52-week trials, adverse reactions led to treatment discontinuation in 4% of Lerochol-treated patients, comparable to the placebo group. The most common adverse reactions (2% in Lerochol arm and >1% higher than placebo) included:
- Nasopharyngitis: 15% (vs 14% placebo).
- Injection site reactions: 12% (vs 5% placebo). In the HeFH trial, this rate was higher at 18%. These reactions were the most frequent cause for discontinuation (1% vs 0% placebo).
- Peripheral edema: 2% (vs <1% placebo).
- Diarrhea: 3% (in HeFH trial).
Special Populations
- Pregnancy: Lerochol should be discontinued when pregnancy is recognized. Based on mechanism of action, it may cause fetal harm. Animal data showed measurable serum concentrations in infants, indicating potential placental transfer.
- Pediatric Use: Safety and effectiveness have not been established. The FDA has waived studies for ages 0 to <6 years and deferred studies for ages 6 to <18 years.
- Renal/Hepatic Impairment: No dose adjustment is necessary for mild to moderate renal or hepatic impairment. The drug has not been studied in severe impairment4.
Immunogenicity
Anti-drug antibodies (ADA) were detected in 15.1% of patients in the 52-week trials, with neutralizing antibodies (NAb) present in 22.8% of those ADA-positive patients. However, the FDA review noted no identified clinically significant effect of ADA/NAb on pharmacokinetics, pharmacodynamics, safety, or efficacy.
Dosing and Practical Use
Lerochol is supplied as a 300 mg/1.2 mL single-dose prefilled syringe.
- Standard Dosage: 300 mg administered subcutaneously once monthly.
- Administration:
- Inject into the abdomen or thigh (or upper arm if administered by a caregiver/HCP).
- Allow the syringe to warm to room temperature for at least 30 minutes prior to use.
- Visually inspect for particulate matter; the solution should be clear to slightly opalescent, brownish-yellow to amber.
Missed Doses:
- If missed by <7 days: Administer ASAP and resume original schedule.
- If missed by 7 days: Administer ASAP and start a new monthly schedule based on this date.
Storage:
- Store refrigerated (2oC to 2oC).
- Key Differentiator: May be kept at room temperature (up to 25oC) in the original carton for up to 3 months. Once removed from refrigeration, it must be used within this window or discarded.
Patient and Market Considerations
The approval of Lerochol addresses key “pain points” in the lipid market: storage and frequency.
- Treatment Burden: The once-monthly dosing is less frequent than the standard bi-weekly regimens of some monoclonal antibodies, potentially improving adherence61.
- Lifestyle Impact: The 3-month room temperature stability allows for easier travel and storage, removing the “cold chain anxiety” often reported by patients using biologics626262.
- Administration: The 1.2 mL volume is relatively small for a biologic, designed to minimize injection pain and duration63636363.
Ideal Patient Profile: Lerochol is well-suited for patients with ASCVD or HeFH who require significant LDL-C lowering (>50%) on top of statins, particularly those who travel frequently, have limited refrigerator space, or struggle with the bi-weekly adherence required by first-generation inhibitors64.
Therapeutic Landscape (Comparative Positioning)
Lerochol enters a competitive class dominated by monoclonal antibodies (evolocumab, alirocumab) and siRNA (inclisiran).
| Feature | Lerochol (lerodalcibep) | Monoclonal Antibodies (Repatha/Praluent) | siRNA (Leqvio) |
| Class | Fusion Protein (Adnectin-HSA) | Monoclonal Antibody | siRNA |
| Dosing | Once Monthly (Self) | Every 2-4 Weeks (Self) | Every 6 Months (HCP) |
| Storage | Room temp up to 3 months | Requires Refrigeration | HCP-managed |
| Mechanism | Binds circulating PCSK9 | Binds circulating PCSK9 | Inhibits PCSK9 synthesis |
Differentiation: Unlike monoclonal antibodies that typically require continuous refrigeration, Lerochol’s room temperature stability is a significant logistical advantage68. While inclisiran offers the lowest dosing frequency (bi-annually), it requires HCP administration. Lerochol offers a “middle ground” of patient autonomy with less frequent dosing than mAbs and greater storage flexibility.
Combination Potential: Early data from the LIBerate-VI study (Phase 3b) suggests that patients switching from inclisiran to Lerochol achieved substantial additional LDL-C reductions (30-40%), indicating a potential role for Lerochol in refractory patients or as part of a sequential therapy strategy.
Lerochol FDA Approval: Regulatory and Post-Approval Perspective
- Approvals: FDA BLA 761427 approved on Dec 12, 2025.
- Advisory Committee: The application was not referred to an advisory committee, as no unexpected safety/efficacy issues were identified.
- Post-Marketing Requirements (PMRs):
- Study 4928-1: A double-blind, placebo-controlled study to assess efficacy and safety in pediatric patients (ages 6 to <18) with HeFH. Final report due October 2028.
- Study 4928-2 (Commitment): “Aged” stability studies on commercial batches to confirm long-term product quality.
- Global Status: EMA decision anticipated in June 2026.
Conclusion
The Lerochol FDA approval (lerodalcibep-liga) validates the fusion protein approach to PCSK9 inhibition, offering a potent, stable, and patient-centric option for lipid management. With efficacy comparable to established biologics (-50% to -59% LDL-C reduction) and a safety profile free of unexpected signals, Lerochol is positioned to improve adherence through its monthly dosing and room-temperature stability. Its role will likely be prominent among active patients and those facing logistical barriers with cold-chain biologics. Future data from pediatric trials and real-world cardiovascular outcomes will further define its long-term place in the therapeutic armamentarium.
Planning a new pharmaceutical manufacturing unit or upgrading for regulatory compliance? Laafon Galaxy provides expert consultancy for WHO-GMP, USFDA compliance, and plant setup. Partner with us to navigate complex regulatory landscapes and build world-class facilities. Get a Free Consultation
FAQs
What is the FDA-approved indication for Lerochol?
Lerochol is indicated as an adjunct to diet and exercise to reduce low-density lipoprotein cholesterol (LDL-C) in adults with hypercholesterolemia, including heterozygous familial hypercholesterolemia (HeFH).
What are the most common side effects of Lerochol?
The most common adverse reactions (ge1%) are injection site reactions, nasopharyngitis, diarrhea, nausea, and peripheral edema
How effective is Lerochol at lowering cholesterol?
In pivotal Phase 3 trials, Lerochol demonstrated sustained LDL-C reductions of approximately 50% to 59% compared to placebo in patients with ASCVD or HeFH80
Can Lerochol be used during pregnancy?
Lerochol should be discontinued if pregnancy occurs. Animal data suggest the drug can cross the placenta, and it may cause fetal harm based on its mechanism of action.
Disclaimer: For informational purposes only. Not a substitute for professional medical advice. Always consult the full prescribing information.
References
- LIB Therapeutics, Inc. (2025, December). LEROCHOL™ (lerodalcibep-liga) injection, for subcutaneous use: Full Prescribing Information. U.S. Food and Drug Administration.
- U.S. Food and Drug Administration. (2025, December 12). BLA Approval Letter – BLA 761427/Original 1 (Lerochol). Center for Drug Evaluation and Research.
- LIB Therapeutics, Inc. (2025). Briefing Document: FDA Approval and Clinical Profile of Lerochol (lerodalcibep-liga).
- LIB Therapeutics, Inc. (2025). Lerochol (lerodalcibep-liga): A Profile of a Third-Generation PCSK9 Inhibitor.
- LIB Therapeutics, Inc. (2025). Understanding Lerochol: A Beginner’s Guide to the Science of PCSK9 Inhibition.
- Study of Efficacy and Safety of LIB003 in Patient With CVD on Statins Requiring Additional LDL-C Reduction (LIBerate-CVD)
- Study to Assess the Efficacy and Safety of LIB003 in HeFH Patients on Oral Lipid Therapy Needing Further LDL-C Reduction (LIBerate-FH)