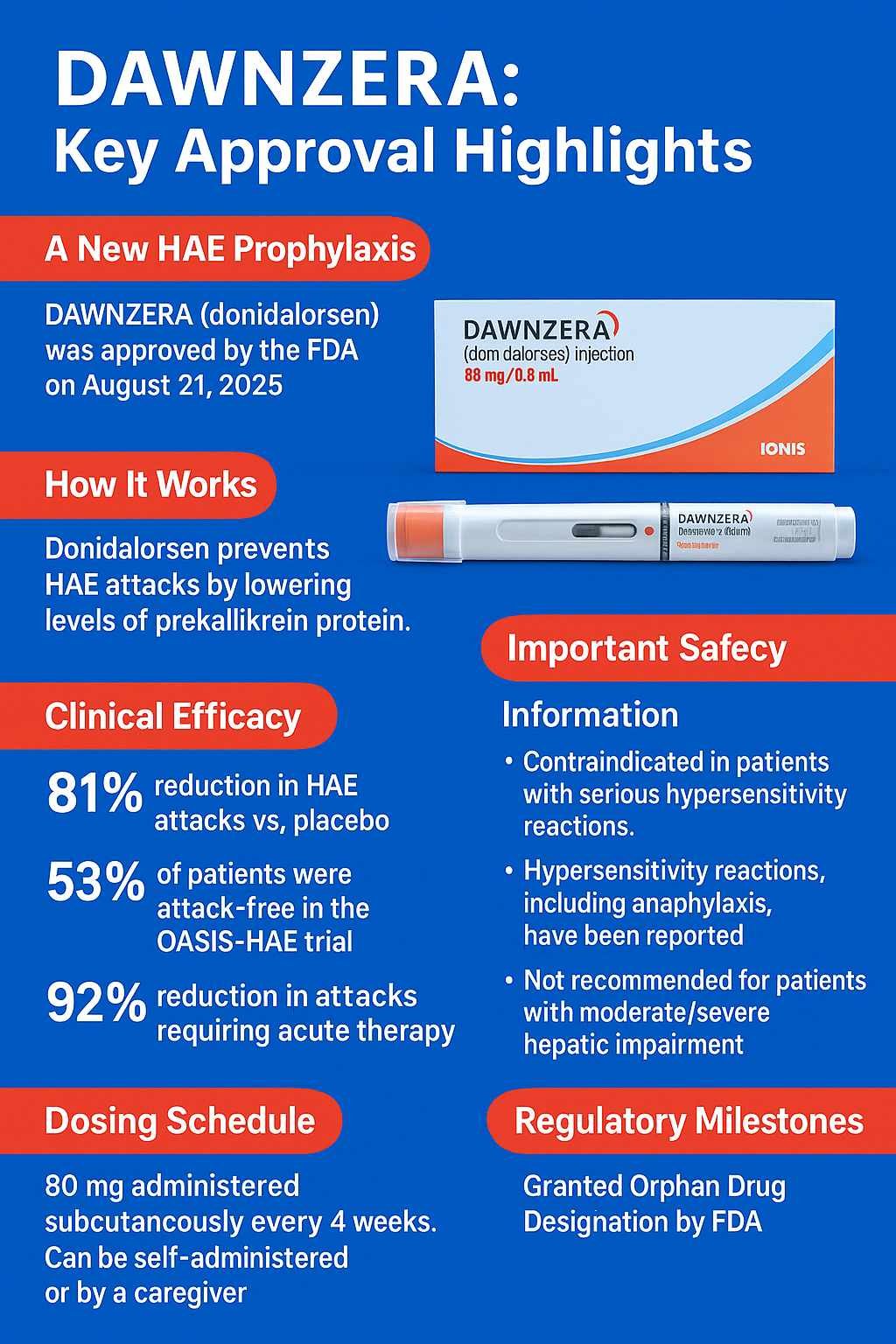
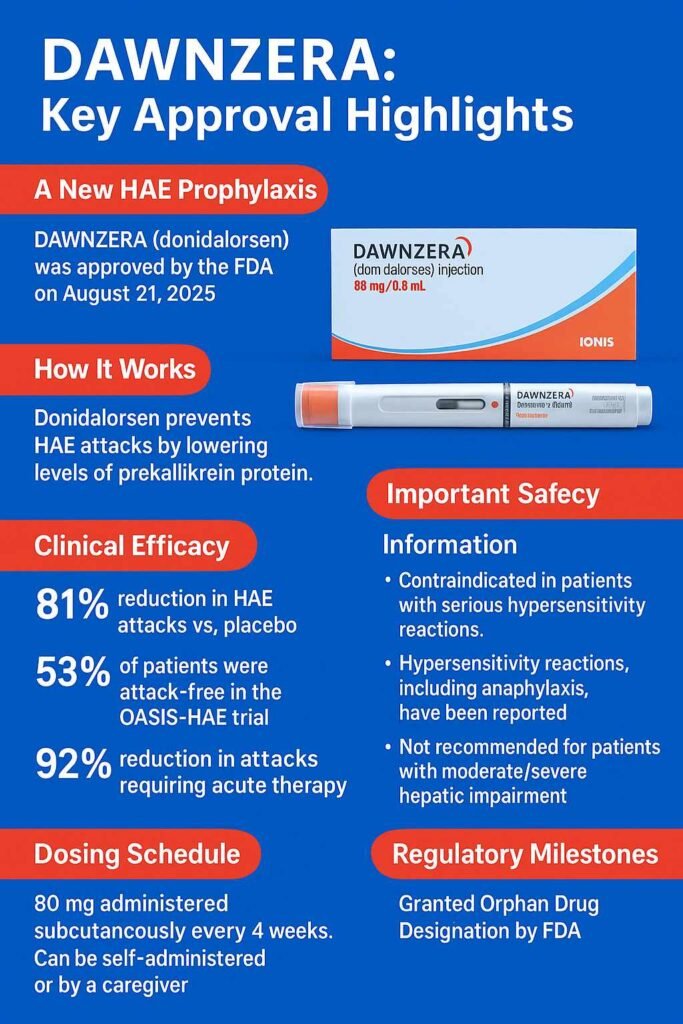
DAWNZERA (donidalorsen) is an FDA-approved antisense oligonucleotide indicated for prophylaxis of hereditary angioedema (HAE) in adults and adolescents aged 12 years and older. Approved on 2025-08-21, DAWNZERA targets prekallikrein mRNA, reducing bradykinin production, the key mediator of angioedema attacks. In the pivotal OASIS-HAE trial (NCT05139810), donidalorsen 80 mg every 4 weeks reduced attack rates by 81% compared with placebo (0.44 vs 2.26 attacks/4 weeks, p<0.001). At this dose, 53% of patients remained attack-free over 24 weeks compared with 9% on placebo. The most common adverse events were injection site reactions (24%), upper respiratory tract infection (9%), and urinary tract infection (9%). Hypersensitivity including anaphylaxis was observed, requiring treatment discontinuation.
No dose adjustment is needed in mild renal or hepatic impairment, but use is not recommended in moderate-to-severe hepatic impairment. The drug carries orphan designation for HAE. Ionis Pharmaceuticals developed DAWNZERA, supplied as an 80 mg/0.8 mL single-dose autoinjector stored at 2–8°C. This article reviews indications, clinical trial data, safety, pharmacology, and regulatory context for healthcare professionals.
Also Read: ANDEMBRY (Garadacimab-gxii): A New Dawn in Preventing Hereditary Angioedema Attacks
Table of Contents
Overview of the Drug
DAWNZERA (donidalorsen) is a prekallikrein-directed antisense oligonucleotide (ASO-GalNAc conjugate). By binding prekallikrein (PKK) mRNA, it reduces PKK protein synthesis and lowers plasma kallikrein activity, ultimately decreasing bradykinin production. Excess bradykinin is central to HAE pathophysiology, leading to recurrent swelling episodes that may be life-threatening.
DAWNZERA represents the first FDA-approved antisense oligonucleotide for HAE prophylaxis. It is intended for subcutaneous self-administration using an autoinjector. The FDA granted approval under NDA 219407 on 2025-08-21.
Indications & Patient Selection
DAWNZERA is approved for:
- Prophylaxis to prevent HAE attacks in adults and pediatric patients ≥12 years.
Patient selection criteria (per label and OASIS-HAE trial):
- Confirmed HAE type I or II
- History of recurrent attacks (≥2 during an 8-week run-in period)
- Exclusion: patients <12 years, history of anaphylaxis to donidalorsen, moderate/severe hepatic impairment
Dosing & Administration
- Recommended dose: 80 mg subcutaneously every 4 weeks
- Alternative dose: 80 mg every 8 weeks may be considered in some patients
- Missed dose: administer as soon as possible, resume regular schedule
- Administration: Abdomen or thigh (self), upper arm (if caregiver)
- Preparation: Remove autoinjector from refrigerator 30 min before use; do not freeze or heat
Storage: 2–8°C; stable for 6 weeks at ≤30°C; shelf life 30 months.
Mechanism of Action
- Donidalorsen is an ASO-GalNAc conjugate that triggers RNase H1-mediated degradation of PKK mRNA.
- This reduces PKK protein, thereby lowering plasma kallikrein and bradykinin generation.
- The result is fewer angioedema attacks in HAE patients with C1-INH deficiency/dysfunction.
Also Read: EKTERLY (Sebetralstat): First Oral HAE Treatment for Rapid Relief During Acute Attacks
Clinical Evidence
OASIS-HAE (NCT05139810)
- Design: 24-week, multicenter, randomized, double-blind, placebo-controlled trial
- Population: 90 patients with HAE (≥12 years); ≥2 attacks during run-in
- Arms: Donidalorsen 80 mg q4wks (n=45), 80 mg q8wks (n=23), placebo (n=22)
Primary Endpoint: Attack rate per 4 weeks
- q4wks: 0.44 vs 2.26 placebo (−81%, 95% CI −89, −65; p<0.001)
- q8wks: 1.02 vs 2.26 placebo (−55%, 95% CI −74, −22; p=0.004)
Secondary Endpoints:
- Attack-free: 53% q4wks vs 9% placebo
- Moderate/severe attack rate reduction: 89% q4wks vs placebo
- Need for acute therapy reduced by 92% (q4wks)
Safety Profile
Common Adverse Events (≥5%):
- Injection site reactions: 24% (q4wks), 5% (q8wks), 4% placebo
- URTI: 9%
- UTI: 9% (q4wks), 0% (q8wks)
- Abdominal discomfort: 7%
Warnings:
- Hypersensitivity including anaphylaxis (serious, requires discontinuation)
- Platelet count reduction: mean −9.6% (q4wks) vs −1.4% placebo
- Liver enzyme increases: usually <3× ULN
Immunogenicity:
- ADA incidence: 20–22% at 24 weeks; 35% in long-term extension
Special Populations
- Pediatrics: Established ≥12 years; not studied <12
- Geriatrics: Insufficient data ≥65 years
- Pregnancy: No human data; animal studies show no harm at 5× MRHD
- Lactation: Unknown in humans; excreted in mice milk
- Renal impairment: Safe in mild impairment; not studied in moderate/severe
- Hepatic impairment: Safe in mild; not recommended in moderate/severe
Contraindications & Interactions
- Contraindications: Serious hypersensitivity to donidalorsen or excipients
- Drug interactions: No clinical DDI studies; not CYP substrate/inhibitor. Metabolite M8 interacts with CYP3A4, BSEP, OATP1B3, MATE1 (in vitro).
Pharmacokinetics & Dynamics
- Tmax: ~2 h
- Cmax: 417 ng/mL (q4wks)
- AUC: ~5240 ng·h/mL
- Half-life: ~1 month (elimination); ~5 h (distribution)
- Distribution: Liver, kidney cortex; >98% protein bound
- Excretion: <1% unchanged in urine
Regulatory Path & Milestones
- NDA 219407 approved: 2025-08-21
- Orphan designation: Granted
- Advisory committee: Not required
- Postmarketing requirements:
- Ongoing liver injury assessment (trials ISIS 721744-CS3, CS7)
- Rat carcinogenicity study
Global Context
- EMA status: Not approved till August 2025
- CDSCO status (India): Not approved till August 2025
Practical Considerations
- Autoinjector format simplifies administration for patients and caregivers
- Room temperature stability for 6 weeks supports home use
- Monitoring: Hypersensitivity, platelet count, liver enzymes
Limitations & Evidence Gaps
- No data in pediatrics <12 years
- Limited geriatric experience
- Long-term safety (liver, carcinogenicity) under postmarketing studies
- EMA/CDSCO regulatory status pending
FAQ
What is DAWNZERA and how does it work to prevent hereditary angioedema attacks?
DAWNZERA (donidalorsen) is the first RNA-targeted therapy approved for HAE prophylaxis. It is an antisense oligonucleotide that binds to prekallikrein (PKK) mRNA, promoting its degradation and reducing bradykinin production, the key mediator of HAE attacks.
What is the recommended dosing schedule for DAWNZERA and how is it administered?
The recommended dose is 80 mg administered subcutaneously every 4 weeks, with the option to consider every 8‑week dosing in suitable patients. It comes in a single-dose autoinjector and can be self-administered into the abdomen, thigh, or upper arm (by caregiver).
What efficacy results supported FDA approval of DAWNZERA?
In the Phase 3 OASIS‑HAE trial, DAWNZERA given every 4 weeks reduced monthly HAE attacks by 81% compared with placebo (attack rate 0.44 vs 2.26; p < 0.001). It also significantly increased the proportion of attack‑free patients.
What are the most common side effects and key safety concerns with DAWNZERA?
Common adverse events include injection site reactions (~24%), upper respiratory infections, urinary tract infections, and abdominal discomfort. Serious hypersensitivity reactions, including anaphylaxis, have been reported and require discontinuation.
Is DAWNZERA approved for children, and what patient populations should exercise caution?
Yes, DAWNZERA is approved for patients 12 years and older. It is not studied or approved in children under 12. Use is not recommended in moderate or severe hepatic impairment, and clinical data in geriatrics (≥65 years) is limited.
Summary
DAWNZERA (donidalorsen) is a new medicine approved in August 2025 to help prevent attacks of hereditary angioedema (HAE), a rare condition that causes sudden swelling in the skin, stomach, or airways. People with HAE often live with unpredictable, painful, and sometimes dangerous attacks.
DAWNZERA works by lowering a protein called prekallikrein, which helps reduce bradykinin, a substance that triggers swelling in HAE. By reducing bradykinin, DAWNZERA helps prevent attacks before they happen.
The medicine is given as a small injection under the skin once every 4 weeks. Some people may use it every 8 weeks. Patients or caregivers can give the injection at home using an autoinjector pen.
In a large study, patients taking DAWNZERA every 4 weeks had about 80% fewer attacks compared with those who received a placebo. More than half of patients taking the medicine every 4 weeks had no attacks at all during the 6-month study.
The most common side effects are mild, such as redness or pain where the injection is given. Rarely, serious allergic reactions can happen.
DAWNZERA offers a new option for people living with HAE, making it easier to manage the condition and reduce attacks.
Third-Party Manufacturing Guidance
👉 Compare your options with our complete guide on third-party pharma manufacturing in India.
References
- U.S. Food and Drug Administration (FDA) – Drugs@FDA Database
https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/219407s000lbl.pdf - ClinicalTrials.gov – OASIS-HAE Trial (NCT05139810)
https://clinicaltrials.gov/study/NCT05139810 - Ionis Pharmaceuticals – DAWNZERA Product Page
https://www.ionis.com/medicines/dawnzera - Medscape Reference – DAWNZERA (donidalorsen) Drug Monograph
https://reference.medscape.com/drug/dawnzera-donidalorsen-4000526 - Angioedema News – Donidalorsen Updates
https://angioedemanews.com/donidalorsen - Reuters – FDA Approval Coverage (Aug 2025)
https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-approves-ionis-drug-rare-genetic-disorder-2025-08-21 - Hereditary Angioedema Association (HAEA) – About HAE
https://www.haea.org/ - FDA Approval Letter – DAWNZERA (donidalorsen), NDA 219407. Reference ID 5646654, 2025-08-21.
- DAWNZERA Prescribing Information. Ionis Pharmaceuticals. Revised 2025-08. Reference ID 5646654.