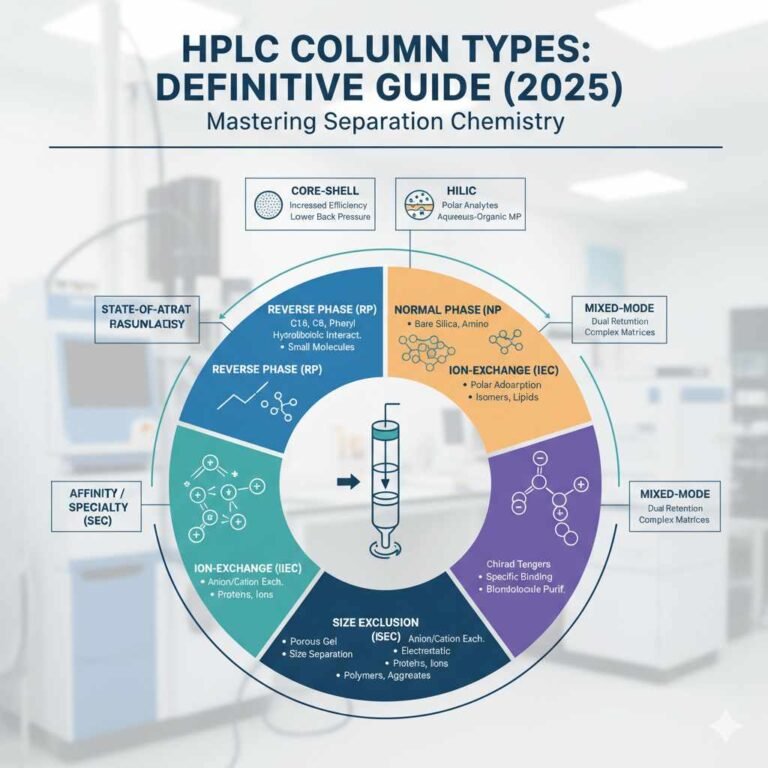
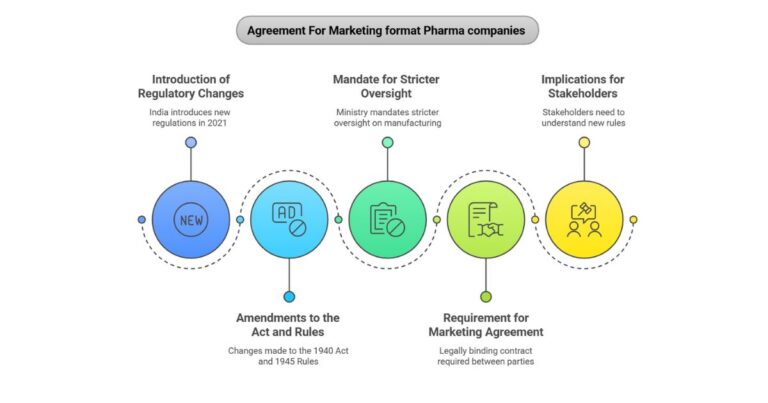
Lycome Plus Capsules | Lycopene Multivitamin & Multimineral Capsules

Lycopene multivitamin & multimineral capsules mainly contain Lycopene as a pillar ingredient. Lycopene is a carotenoid that is a type of plant pigment. It has antioxidant properties. Usually, it is found in tomatoes and tomato products including ketchup, pasta sauce,…