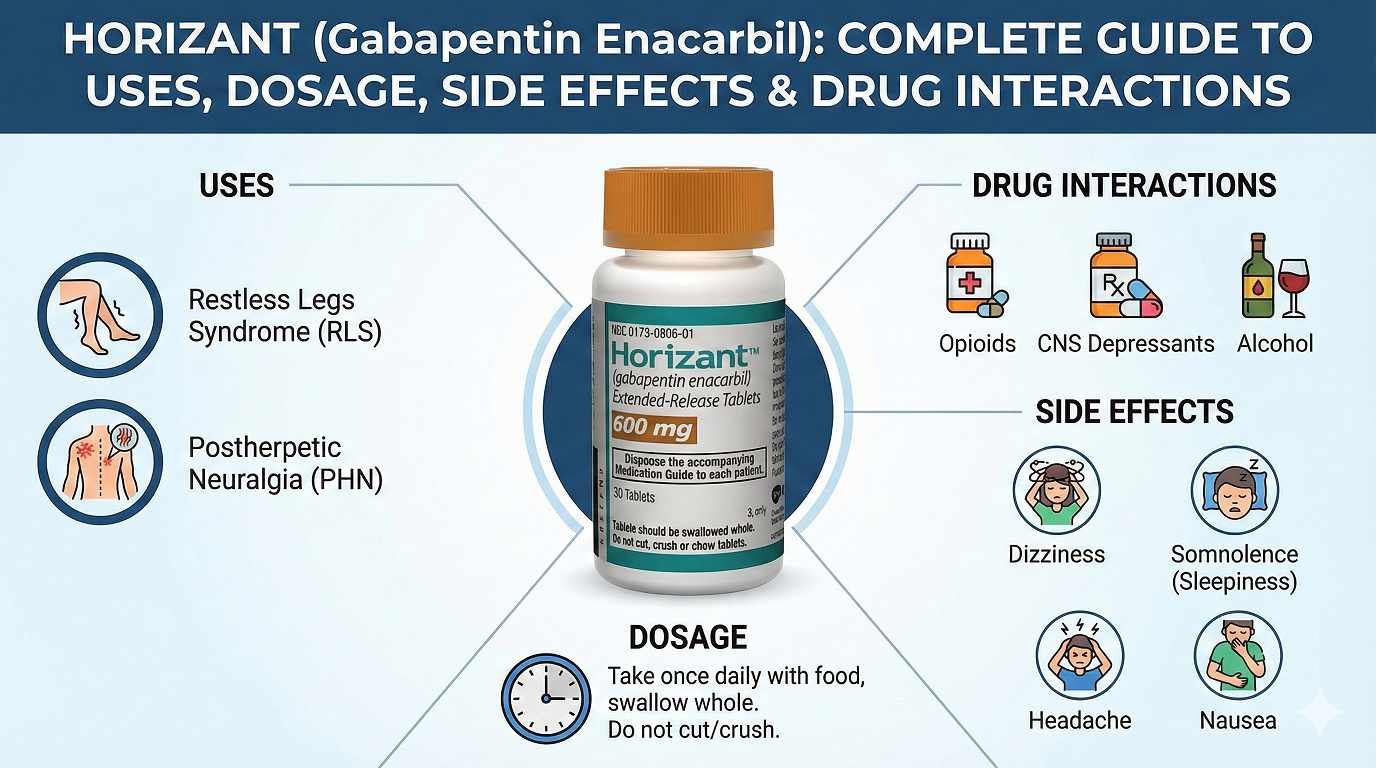
Horizant (gabapentin enacarbil) is an FDA-approved extended-release medication used to treat two serious neurological conditions: moderate-to-severe restless legs syndrome (RLS) and postherpetic neuralgia (PHN)—the debilitating nerve pain that follows shingles infection. Unlike standard gabapentin which requires three daily doses, Horizant offers a more convenient once-daily formulation for RLS patients, improving treatment adherence and quality of life. Approved by the FDA in 2011 for RLS and 2012 for PHN, this medication has become a first-line treatment option as clinical guidelines have evolved significantly.

What Is Horizant? Understanding the Medication
Horizant is the brand name for gabapentin enacarbil, an extended-release anticonvulsant medication belonging to the gabapentinoid class. It represents a significant advancement over immediate-release gabapentin because it uses a specialized absorption mechanism called the LAT1 transporter, which provides superior bioavailability and more consistent drug levels throughout the day.
Here’s what makes Horizant different: gabapentin enacarbil is a “prodrug”—meaning your body converts it to gabapentin after absorption. This clever design allows Horizant to overcome a key limitation of regular gabapentin: when taken at high doses, standard gabapentin becomes poorly absorbed as its transporters become saturated. Horizant bypasses this saturation limitation, delivering approximately 74% bioavailability compared to only 27-60% for immediate-release gabapentin. This superior absorption translates to more predictable pain relief and symptom control.
The medication comes as 600 mg extended-release tablets and is manufactured by GlaxoSmithKline in partnership with XenoPort Inc., the company that developed the proprietary absorption technology.
Conditions Treated: RLS and Postherpetic Neuralgia
Restless Legs Syndrome (RLS)
Restless legs syndrome is a neurological disorder characterized by an irresistible urge to move the legs, typically accompanied by uncomfortable sensations like tingling, burning, or aching. Symptoms worsen during periods of inactivity—especially at night while lying in bed—which severely disrupts sleep and diminishes quality of life. An estimated 5-10% of the population experiences RLS, making it far more common than many realize.
Horizant is particularly effective for RLS because of its once-daily dosing at 5 PM. This timing aligns perfectly with when RLS symptoms typically peak (evening and night), ensuring medication levels are highest when patients need relief most. Clinical trials demonstrated that 600 mg once daily significantly reduces the urge to move and improves sleep quality in patients with moderate-to-severe disease. Recent 2025 treatment guidelines from the American Academy of Sleep Medicine have elevated gabapentinoids like Horizant to first-line status, moving away from older dopamine agonist medications that carry risks of augmentation (worsening of symptoms over time).
Postherpetic Neuralgia (PHN)
Postherpetic neuralgia is chronic nerve pain persisting after a shingles rash heals. Unlike the acute pain of shingles itself, PHN can last months or years, sometimes indefinitely. Patients describe it as burning, shooting, or electric shock sensations that significantly impair daily functioning, mood, and sleep. PHN affects approximately 10-18% of shingles patients, and risk increases substantially with age.
Horizant is FDA-approved for PHN at a dose of 600 mg twice daily (started at once daily, then titrated), providing significant pain reduction in clinical trials. The medication’s mechanism—reducing abnormal nerve firing—directly addresses the underlying neurophysiology of PHN, making it one of the few medications with strong clinical evidence for this difficult-to-treat condition.
How Horizant Works: Mechanism of Action
Horizant belongs to the anticonvulsant or anti-seizure drug class, though its therapeutic effect in RLS and PHN doesn’t involve seizure prevention. Instead, it works through a different mechanism: gabapentin reduces abnormal electrical activity in damaged or sensitized nerves, thereby decreasing pain perception and the neural drive to move the legs.
Specifically, Horizant is believed to work by:
- Modulating calcium channels at nerve endings, reducing the release of excitatory neurotransmitters (chemical messengers that amplify pain signals)
- Increasing GABA production, promoting the brain’s natural calming neurotransmitter activity
- Reducing neuronal hyperexcitability, particularly in the spinal cord dorsal horn—the area that processes pain and sensory information
The extended-release formulation maintains consistent medication levels throughout 24 hours, providing steady symptom control without the fluctuations that occur with immediate-release gabapentin dosed three times daily. This stability is why many patients report better overall efficacy and improved sleep quality with Horizant.
Horizant Dosage and Administration: Getting It Right
Proper dosing is critical for both efficacy and safety. It’s equally important to understand that Horizant must be taken with food—not on an empty stomach.
Correct Dosage for Restless Legs Syndrome
Standard RLS Dose: 600 mg once daily at approximately 5 PM, taken with food.
This single daily dose is the FDA-approved regimen. Do not increase beyond 600 mg daily for RLS—clinical trials demonstrated no additional benefit at higher doses, and increased dosing simply leads to more side effects. The specific 5 PM timing matters because it positions peak medication levels to coincide with when RLS symptoms naturally worsen (evening and night).
Correct Dosage for Postherpetic Neuralgia
PHN Titration Schedule:
- Days 1-3: 600 mg once daily in the morning with food
- Day 4 onward: 600 mg twice daily (morning and evening with food)
Unlike RLS where you immediately start at the full dose, PHN treatment requires a three-day titration period. This gradual approach minimizes early side effects while your body adjusts. Peak effectiveness typically develops within 4-8 weeks of consistent treatment at the full 1,200 mg daily (600 mg twice daily) dose.
Critical: Why You Must Take Horizant with Food
Horizant’s superior bioavailability depends on food—specifically, meals containing fat. When taken with a fat-containing meal, Horizant achieves approximately 74% bioavailability. Taking it on an empty stomach substantially reduces absorption, meaning you’ll receive significantly less medication than intended. Appropriate meals include:
- Breakfast with eggs, butter, or oil-based dishes
- Lunch with fish, nuts, or fatty meats
- Dinner with oil-based cooking or fatty protein sources
- Even a small snack with cheese or peanut butter helps
If you forget to take Horizant with food, take your next dose at the scheduled time with food. Do not double dose to compensate.
Dose Adjustments for Kidney Function
If you have reduced kidney function (estimated glomerular filtration rate below 60 mL/min), your doctor may need to adjust your dose. Horizant is eliminated through the kidneys, so impaired renal function increases medication accumulation. Always inform your healthcare provider of any kidney problems before starting Horizant.
Side Effects: What to Expect and When
Understanding potential side effects helps you distinguish normal adjustment reactions from dangerous warning signs requiring medical attention.
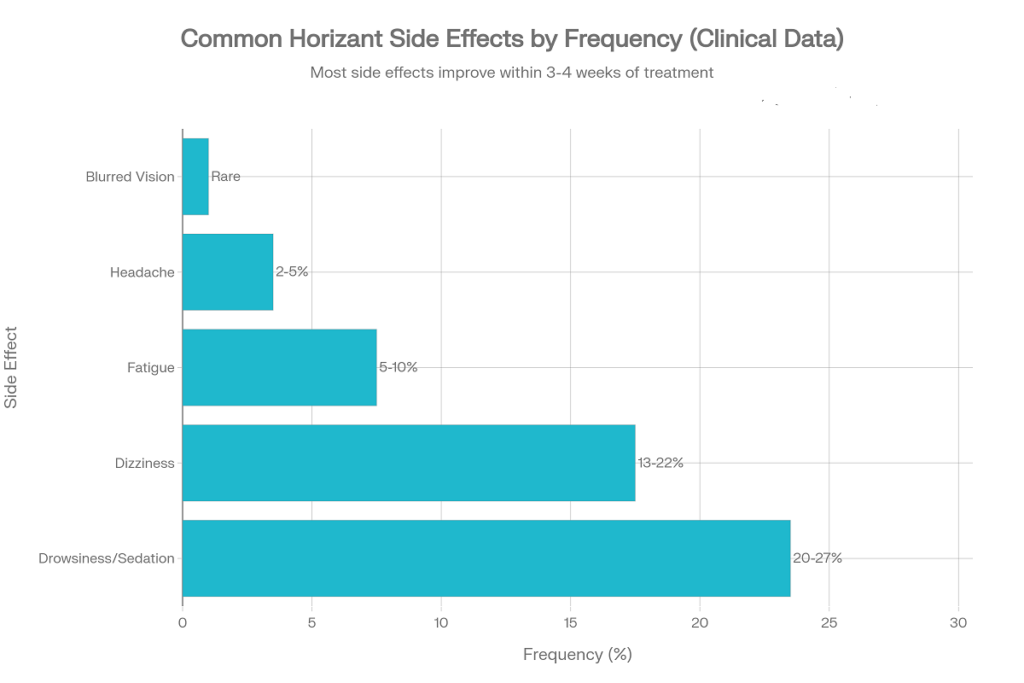
Most Common Side Effects
When starting Horizant at the recommended RLS dose (600 mg), the most frequently reported side effects are:
| Side Effect | Frequency | Typical Onset | Duration |
|---|---|---|---|
| Dizziness | 13-22% of patients | First 1-2 weeks | Often improves by week 3-4 |
| Drowsiness/Sedation | 20-27% of patients | First dose | Frequently decreases with continued use |
| Fatigue | 5-10% of patients | First week | Variable; usually temporary |
| Headache | 2-5% of patients | Variable | Usually resolves within days |
| Blurred Vision | Rare | Variable | Temporary in most cases |
These side effects occur because Horizant slows nervous system activity. Importantly, your body often develops tolerance over 2-4 weeks as your nervous system adjusts, and drowsiness typically improves significantly. Taking your dose at 5 PM rather than morning minimizes daytime impairment—you’ll sleep through the worst of the sedation.
Managing Common Side Effects:
- Avoid driving or operating machinery during the first 2-4 weeks until you know your individual response
- Do not consume alcohol (see section below on interactions)
- Stay hydrated and maintain adequate nutrition
- Rise slowly from sitting or lying positions to minimize dizziness
- If side effects don’t improve after 4 weeks, contact your doctor about dose adjustment
Serious Side Effects Requiring Immediate Medical Attention
While rare, some side effects are serious and demand immediate action:
Suicidal Thoughts or Mood Changes: As an antiepileptic drug (AED), Horizant carries a black box warning for increased risk of suicidal thoughts and behaviors. Although this occurs in less than 0.1% of patients, it’s a recognized risk. Seek emergency care immediately if you experience suicidal ideation, new or worsening depression, anxiety, unusual mood changes, or behavioral changes. Family members should monitor for these warning signs, particularly during the first few weeks of treatment.
Respiratory Depression: Particularly when combined with opioids or other CNS depressants (discussed below), Horizant can slow breathing to dangerous levels. Seek emergency care if you experience difficulty breathing, shallow breathing, or loss of consciousness.
Severe Allergic Reactions: Though uncommon, anaphylaxis can occur. Seek emergency care for facial swelling, throat closure, difficulty breathing, or severe rash.
Seizures Upon Abrupt Discontinuation: Do not stop Horizant suddenly. Abrupt withdrawal can trigger seizures even in people without seizure disorders. Always taper under medical supervision.
Horizant Drug Interactions: Critical Safety Information
Horizant interacts with numerous medications and substances. Informed communication with your healthcare provider about everything you take is essential for safe treatment.
Critical Interactions (Avoid or Require Physician Management)
Alcohol: Alcohol significantly increases Horizant absorption, converting extended-release kinetics to near-immediate-release levels. This causes dangerous spikes in sedation, dizziness, impaired cognition, and respiratory depression. Additionally, alcohol itself is a CNS depressant, creating synergistic effects. Complete avoidance of alcohol is mandatory during Horizant treatment—even moderate consumption is unsafe.
Opioid Medications (Morphine, Codeine, Hydrocodone, Oxycodone): Combining Horizant with opioids significantly increases risk of severe sedation, respiratory depression, coma, and even death. Postmarketing reports have documented fatal respiratory depression from this combination. If you require opioids, your physician must carefully supervise—many doctors now avoid this combination entirely. If concurrent use is unavoidable, respiratory monitoring and naloxone availability are essential.
Marijuana/Cannabis: THC compounds Horizant’s CNS effects, amplifying dizziness, sedation, cognitive impairment, and motor incoordination. While cannabis may enhance pain relief, it simultaneously worsens side effects. Do not drive or operate machinery if combining these substances. Discuss with your doctor whether cannabis use is safe in your individual situation.
Benzodiazepines (Alprazolam, Lorazepam, Clonazepam): Similar to opioids, benzodiazepines cause additive CNS depression and respiratory depression risk. Use only under close medical supervision, if at all.
Moderate Interactions (Requires Caution and Monitoring)
Antihistamines (Diphenhydramine, Cetirizine): Over-the-counter cold and allergy medications containing antihistamines add to Horizant’s sedating effects, potentially causing excessive drowsiness. Use caution; inform your doctor if using regularly.
Muscle Relaxants (Carisoprodol, Cyclobenzaprine): These agents enhance CNS depression. Use only under medical supervision.
Other Gabapentin Formulations: Never combine Horizant with immediate-release gabapentin or other gabapentin products—this creates overdose risk.
Always Inform Your Healthcare Provider About:
- All prescription medications
- Over-the-counter drugs (especially antihistamines, pain relievers, sleep aids)
- Herbal supplements and vitamins
- Alcohol or recreational substance use
- Medical conditions, particularly respiratory disease, kidney disease, depression, or history of substance use disorder
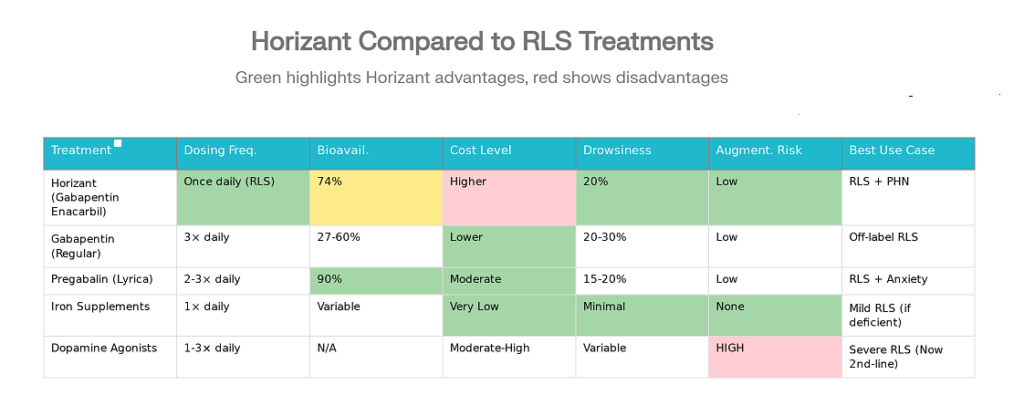
Horizant vs. Other RLS Treatments: How It Compares
Understanding how Horizant compares to alternative RLS treatments helps you and your doctor make informed decisions.
| Feature | Horizant | Regular Gabapentin | Pregabalin (Lyrica) | Iron Supplements | Dopamine Agonists |
|---|---|---|---|---|---|
| FDA Approved for RLS | Yes (2011) | No (off-label) | Yes | Yes (if deficient) | Yes (older, de-emphasized) |
| Dosing Frequency | Once daily (RLS) | 3× daily | 2-3× daily | 1× daily | 1-3× daily |
| Bioavailability | 74% (consistent) | 27-60% (variable) | 90% | Variable | N/A |
| Cost | Higher | Lower | Moderate | Very low | Moderate-High |
| Drowsiness Rate | 20% | 20-30% | 15-20% | Minimal | Variable |
| Augmentation Risk | Low | Low | Low | None | HIGH |
| Best For | RLS + PHN together | Mild RLS | RLS with anxiety | Mild RLS with iron deficiency | Severe RLS (now second-line) |
Key Insight: Until recently, dopamine agonists were first-line RLS treatment. However, 2025 treatment guidelines have shifted away from these due to augmentation—a phenomenon where symptoms worsen over time, requiring dose escalation in a cycle of dependency. Gabapentinoids like Horizant are now recommended as first-line therapy because they don’t cause augmentation and have better long-term safety profiles.
Frequently Asked Questions
-
Q: How long does Horizant take to work?
Most patients notice improvement in RLS symptoms within 1-4 weeks. Peak effectiveness typically develops by week 8. If you experience no improvement after 4-8 weeks of consistent, correct use, inform your doctor—dose adjustment or alternative therapy may be needed.
-
Is Horizant a controlled substance?
No. Horizant is not classified as a controlled substance under the DEA or narcotics laws, meaning it lacks the high abuse potential of opioids or benzodiazepines. However, psychological dependence may develop with long-term use. Never stop Horizant abruptly due to withdrawal seizure risk—always taper under medical supervision.
-
Can Horizant cause addiction?
Horizant does not produce euphoria (“getting high”) and has minimal abuse potential. The real concerns are psychological habituation and withdrawal symptoms if stopped suddenly (seizures, tremor). Always use as prescribed and report any dependence concerns to your doctor.
-
Does Horizant make you sleepy?
Yes, drowsiness and sedation are common, particularly during the first 2-4 weeks. Taking your dose at 5 PM allows you to sleep through the worst effects. Drowsiness often improves significantly as your body adjusts, but some patients remain sedated. Inform your doctor if drowsiness persists beyond 4 weeks.
-
Can I drive after taking Horizant?
Do not drive during the first 2-4 weeks while your body adjusts. Even afterward, Horizant causes dizziness and impaired cognition similar to moderate alcohol intoxication. Assess your individual response before driving. Never drive if you feel drowsy or dizzy.
-
What if I miss a dose?
For RLS: Take your missed dose only if it’s within 12 hours. If more than 12 hours have passed, skip that dose and resume your normal schedule the next day. Never double-dose.
-
Is Horizant safe during pregnancy?
Horizant is Category C—animal studies show no fetal harm, but human pregnancy studies are limited. Untreated RLS can harm pregnancy outcomes. If you’re pregnant or planning pregnancy, discuss risks versus benefits with your doctor. Do not stop abruptly due to withdrawal seizure risks.
-
Can I take Horizant with food supplements or vitamins?
Generally yes, but inform your doctor about all supplements. Orlistat (weight loss medication) may reduce Horizant absorption. Iron supplements don’t directly interact but may reduce RLS if you’re deficient—ask your doctor about iron testing.
Important Safety Information and Medical Disclaimer
⚠️ MEDICAL DISCLAIMER: This article provides educational information about Horizant and is not a substitute for professional medical advice. Always consult a licensed healthcare provider before starting, stopping, or changing any medication. Individual responses to Horizant vary considerably. If you experience suicidal thoughts, mood changes, unusual behavior, or respiratory difficulties, seek emergency medical care immediately by calling 911 or going to the nearest emergency room.
This content is based on FDA prescribing information and current clinical guidelines as of January 2026. Always obtain your complete prescription information from your pharmacist and read the medication guide provided with your Horizant prescription.
Article Information:
- Last Updated: January 24, 2026
- Reviewed By: Dr. Yogesh Chaudhary (B.Pharma), Senior Pharmacist, S.N. Medical College, Agra, Uttar Pradesh
- Fact-Checked: January 24, 2026
- Sources: FDA Prescribing Information, American Academy of Sleep Medicine RLS Guidelines, Clinical Trial Data, Mayo Clinic, MedicineNet
Disclaimer: All information on this site is for educational purposes only. The information provided should not be used for diagnosis or treatment of any health condition without professional medical advice. Always consult a qualified healthcare practitioner for medical examination and treatment.
Reviewed by;

Dr. Yogesh Chaudhary (B. Pharma)
Senior Pharmacist at S.N. Medical College, Agra-(UP)
References
- Gabapentin enacarbil – Wikipedia https://en.wikipedia.org/wiki/Gabapentin_enacarbil
- Gabapentin Enacarbil for PHN – EMPR https://www.empr.com/home/mpr-first-report/
- 2025 RLS Guidelines Review – Neurology Live https://www.neurologylive.com/view/clinical-review-2025-restless-leg-syndrome-guidelines
- GSK/XenoPort FDA Approval 2012 – FiercePharma https://www.fiercepharma.com/pharma/
- Gabapentin Enacarbil Crossover Trial – PubMed https://pubmed.ncbi.nlm.nih.gov/24102928/
- 2025 RLS Guidelines – EMJ Reviews https://www.emjreviews.com/neurology/news/restless-legs-syndrome-updated-clinical-practice-guideline/
- GSK/XenoPort FDA Approval 2011 – GSK Press Release https://www.gsk.com/en-gb/media/press-releases/
- Meta-Analysis Gabapentin PHN – Wiley Online https://onlinelibrary.wiley.com/doi/10.1155/2018/7474207
- AASM RLS Guidelines PDF https://www.irlssg.org/wp-content/uploads/2025/05/
- Horizant Official Prescribing https://horizant.com/hcp/prescribing-horizant/
- MedicineNet Horizant Side Effects https://www.medicinenet.com/side_effects_of_gabapentin_enacarbil/
- Gabapentin and Cannabis Interactions – Presto Doctor https://prestodoctor.com/content/general/gabapentin-and-weed
- FDA HORIZANT NDA Label (Primary) https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022399Orig1s003.pdf
- Leafwell Cannabis/Gabapentin https://leafwell.com/blog/gabapentin-and-weed
- GoodRx Horizant Information https://www.goodrx.com/horizant/what-is
- FDA HORIZANT Label 2011 https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022399s000lbl.pdf
- FDA HORIZANT Label 2020 https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022399s011lbl.pdf
- Biomedres Synergistic Effects Cannabis Gabapentin https://biomedres.us/pdfs/
- YMYL & EEAT for Pharma – Varn Health https://varnhealth.com/industry-insights/
- Pharmacokinetics Gabapentin – PMC https://pmc.ncbi.nlm.nih.gov/articles/PMC5914156/
- Google E-A-T Healthcare – Healthcare Success https://healthcaresuccess.com/blog/healthcare-marketing/
- Healthcare E-E-A-T & YMYL – Hill Web Creations https://www.hillwebcreations.com/healthcare-sites-content-strategy-e-a-t-criteria/
- Bioavailability Variability Gabapentin Enacarbil – PMC https://pmc.ncbi.nlm.nih.gov/articles/PMC9083487/