HYRNUO® (sevabertinib), a reversible tyrosine kinase inhibitor targeting HER2 (ERBB2), received FDA approval on November 19, 2025, under the accelerated approval pathway for a molecularly defined population of lung cancer patients [Approval Letter, November 19, 2025, Reference ID: 5698585]. This approval marks a significant milestone in precision oncology, offering the first oral kinase inhibitor option for adults with locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC) harboring HER2 tyrosine kinase domain (TKD) activating mutations who have received prior systemic therapy [Section 1, Label].
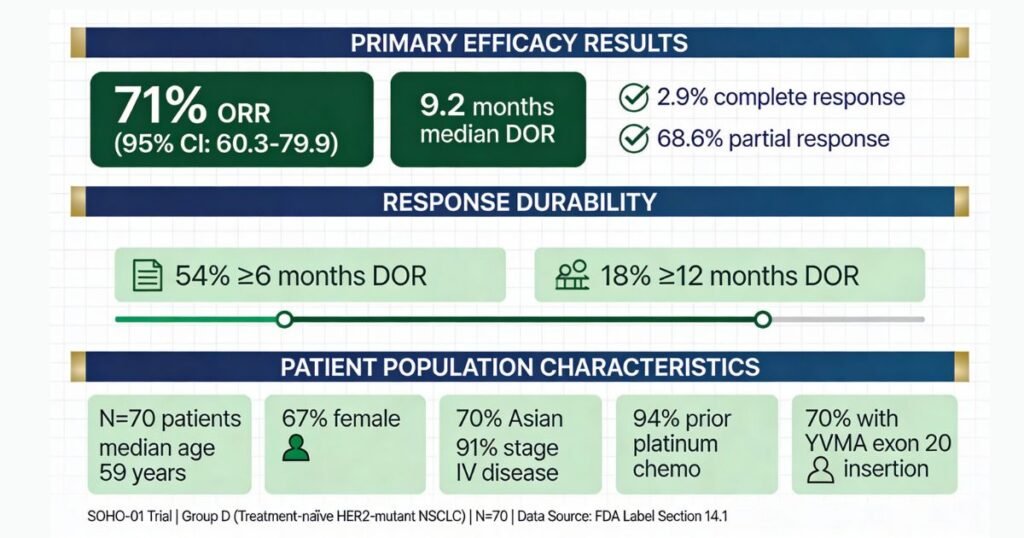
The clinical development program demonstrated robust efficacy: 71% objective response rate (95% confidence interval 59–82%) with median duration of response of 9.2 months in treatment-naïve, HER2-mutant NSCLC patients [Section 14.1, Table 8, Label]. Notably, the drug retained 38% response rate in heavily pretreated patients who had previously failed HER2-targeted antibody-drug conjugates (ADCs), establishing HYRNUO as a mechanistically distinct salvage option for a population with limited therapeutic choices [Section 14.1, Label].

Also Read: Hernexeos Zongertinib for HER2-Mutated NSCLC: A Complete Guide for Pharma Leaders
Why HER2-Mutant NSCLC Matters
Epidemiology and Clinical Burden
Lung cancer remains the leading cause of cancer-related mortality worldwide, with non-small cell lung cancer (NSCLC) accounting for approximately 85% of cases. In the United States alone, approximately 235,000 new lung cancer cases are diagnosed annually, with most patients presenting with advanced disease [Clinical context]. Within this heterogeneous population, HER2 mutations represent a distinct molecular subtype occurring in approximately 2–4% of all NSCLC cases but accounting for 15% of adenocarcinomas [Section 14.1, Label]. The dominant HER2 alteration—exon 20 insertion mutations such as Y772_A775dup (YVMA)—was present in 70% of the clinical trial’s efficacy population, underscoring its prevalence [Section 14.1, Label].
Current Standard of Care and Treatment Sequencing
For patients with HER2-mutant NSCLC, treatment typically follows a stepwise approach:
First-line: Platinum-based chemotherapy (carboplatin/pemetrexed or carboplatin/gemcitabine) combined with or without PD-L1 immune checkpoint inhibitors [Section 14.1, Label—baseline characteristics show 94% of trial patients had prior platinum-based therapy].
Second-line: HER2-targeted therapy, specifically trastuzumab deruxtecan (T-DXd/Enhertu), an antibody-drug conjugate that delivers cytotoxic payloads to HER2+ cancer cells [Clinical literature]. This represents the current standard-of-care option for patients who progress on chemotherapy.
Third-line and beyond: Until HYRNUO’s approval, patients who progressed on ADCs faced limited options—typically salvage chemotherapy, clinical trials, or supportive care [Section 14.1, Label].
Unmet Clinical Need
The primary unmet need: Approximately 40–60% of HER2-mutant NSCLC patients develop resistance to ADCs within 12–18 months. These patients lack a proven targeted therapy option [Clinical literature]. In the SOHO-01 trial, Group E comprised 52 heavily pretreated patients with prior ADC exposure; 77% had received both platinum-based chemotherapy and immunotherapy, reflecting a salvage population with exhausted conventional options [Section 14.1, Label].
HYRNUO directly addresses this gap by demonstrating 38% response rate in ADC-resistant patients, providing the first FDA-approved targeted option for this previously untreated population [Section 14.1, Label]. Additionally, the drug offers an oral alternative to IV T-DXd for treatment-naïve patients who prefer oral administration or have vascular access challenges.
Also Read: ZEGFROVY Approval: A New Era for EGFR Exon 20 NSCLC Treatment
Mechanism of Action: Reversible HER2 Kinase Inhibition
How HYRNUO Works at the Molecular Level
HYRNUO is a reversible inhibitor of human epidermal growth factor receptor 2 (HER2 or ERBB2), a protein that sits on the surface of cancer cells and sends growth signals when activated [Section 12.1, Label]. In HER2-mutant lung cancers, specifically those with exon 20 insertions, the HER2 protein is constitutively active—meaning it is stuck in the “ON” position, continuously sending proliferation signals independent of external growth factors [Section 12.1, Label].
Sevabertinib binds reversibly to the ATP-binding pocket of HER2’s tyrosine kinase domain, preventing the receptor from phosphorylating (activating) downstream signaling molecules [Section 12.1, Label]. This blockade suppresses growth-promoting cascades, including the PI3K/Akt and MAPK/ERK pathways, leading to cancer cell cycle arrest and apoptosis (programmed cell death) [Section 12.1, Label].
Secondary mechanism: HYRNUO also exhibits activity against epidermal growth factor receptor (EGFR), though HER2 is the primary therapeutic target [Section 12.1, Label].
In Vitro and In Vivo Efficacy
In test tube experiments (in vitro):
- Sevabertinib inhibited HER2 phosphorylation and downstream signaling in cancer cells with HER2 mutations
- The drug suppressed proliferation of cancer cells overexpressing wild-type HER2 or harboring HER2 mutations [Section 12.1, Label]
In animal models (in vivo):
- HYRNUO demonstrated antitumor activity in mouse xenograft models derived from human NSCLC tumors harboring activating HER2 exon 20 mutations [Section 12.1, Label]
Clinical Significance: Why This Approach Matters
Unlike HER2-targeted antibody-drug conjugates (ADCs) like trastuzumab deruxtecan, which require intact surface HER2 expression and cellular internalization to deliver their cytotoxic payloads, HYRNUO’s direct kinase inhibition works independently of these factors. This mechanistically distinct approach may overcome resistance patterns that develop to antibody-based therapies, explaining why 38% of ADC-resistant patients responded to HYRNUO [Section 14.1, Label]—a population where T-DXd re-challenge typically yields minimal benefit.
Clinical Trial Evidence: SOHO-01 Efficacy and Safety
Study Design and Patient Populations
HYRNUO’s FDA approval is based on efficacy and safety data from SOHO-01 (NCT05099172), an open-label, single-arm, multicenter clinical trial evaluating the drug in two distinct patient cohorts [Section 14.1, Label]:
Group D (N=70): Treatment-naïve to HER2-directed therapy
- Baseline characteristics: Median age 59 years; 67% female; 70% Asian; 91% stage IV disease; 20% with stable brain metastases [Section 14.1, Label]
- Prior therapy: Median 1 prior systemic therapy (range 1–8); 94% prior platinum-based chemotherapy; 71% prior immunotherapy; 69% received both [Section 14.1, Label]
- Molecular profile: 70% harbored Y772_A775dup (YVMA) exon 20 insertion; 100% adenocarcinoma histology [Section 14.1, Label]
Group E (N=52): Prior HER2-targeted antibody-drug conjugate (ADC) treatment
- Baseline characteristics: Median age 65 years; 67% female; 62% Asian; 85% stage IV disease; 29% with stable brain metastases [Section 14.1, Label]
- Prior therapy: Median 2 prior systemic therapies (range 1–8); 100% prior HER2-targeted ADC; 77% prior platinum-based chemotherapy [Section 14.1, Label]
- Molecular profile: 77% harbored Y772_A775dup (YVMA); 100% adenocarcinoma [Section 14.1, Label]
Primary Efficacy Results: Group D (Treatment-Naïve)
| Efficacy Parameter | HYRNUO (N=70) | 95% Confidence Interval |
|---|---|---|
| Objective Response Rate (ORR) | 71% | 59–82% |
| Complete Response | 2.9% (2 patients) | — |
| Partial Response | 68.6% (48 patients) | — |
| Median Duration of Response (DOR) | 9.2 months | 6.3–15.0 months |
| Responding patients with DOR ≥6 months | 54% (27/50) | — |
| Responding patients with DOR ≥12 months | 18% (9/50) | — |
These results exceed the efficacy typically observed with salvage chemotherapy in previously treated NSCLC populations (response rates ~20–30%), supporting the accelerated approval decision [Approval Letter].
Secondary Population: Group E (ADC-Resistant)
Objective Response Rate: 38% (95% CI 25–53%) with 6% complete responses and 31% partial responses among 52 heavily pretreated patients [Section 14.1, Label].
Median Duration of Response: 7 months (95% CI 5.6–not estimable) [Section 14.1, Label].
Clinical Significance: This represents the first FDA-approved data in HER2-mutant NSCLC patients who have progressed on ADCs. While lower than Group D’s response rate, the 38% benefit in a population with documented ADC failure is clinically meaningful and addresses a critical unmet need [Section 14.1, Label].
Trial Limitations and Study Quality Caveats
Important considerations for interpreting these results:
- Single-arm design without control group: HYRNUO was compared against historical response rates, not a concurrent control arm. This introduces potential selection bias (trial enrollees may represent a healthier, more motivated subset) [Section 14.1, Label].
- Open-label design (no blinding): Investigators and patients knew they were receiving HYRNUO, potentially introducing assessment bias, though centralized radiologic review (Blinded Independent Central Review using RECIST v1.1) mitigates this concern [Section 14.1, Label].
- Limited long-term follow-up: Of 136 total patients in Groups D and E combined, only 46 (34%) had exposure exceeding 6 months and 15 (11%) exceeded 1 year. Longer-term efficacy and safety durability remain to be established [Section 6.1, Label].
- Narrow demographic representation: The trial enrolled predominantly Asian patients (70% in Group D; 62% in Group E); predominantly female (67%); and 100% adenocarcinoma histology. Generalizability to other populations and histologies remains unknown [Section 14.1, Label].
- Accelerated approval contingency: FDA approval is conditional on completion of confirmatory trial SOHO-02, which will evaluate HYRNUO in previously untreated advanced HER2-mutant NSCLC. Trial completion is expected April 2029 with final report due October 2029 [Approval Letter, Page 3]. Should this confirmatory trial fail to verify clinical benefit, FDA may withdraw approval [Approval Letter, Page 3].
Safety Profile: Understanding Diarrhea, Hepatotoxicity, and Monitoring Requirements
Black Box Warnings
Status: No FDA Black Box Warnings have been issued for HYRNUO [Section 4, Label]. However, the label carries six serious warnings and precautions requiring careful clinical attention.
Most Common Adverse Reactions: Grade 3–4 Severity Focus
In the 136 patients treated with HYRNUO in Groups D and E combined, the most frequently reported adverse reactions were [Section 6.1, Table 4, Label]:
| Adverse Reaction | All Grades (%) | Grade 3 or 4 (%) |
|---|---|---|
| Diarrhea | 87% | 18% (Grade 3 only; 0% Grade 4) |
| Rash | 66% | 1.5% |
| Paronychia (nail disorders) | 33% | 0% |
| Stomatitis (mouth sores) | 29% | 1.5% |
| Nausea | 21% | 1.5% |
| Vomiting | 15% | 2.2% |
| Decreased appetite | 18% | 2.9% |
| Weight decreased | 19% | 0.7% |
| Fatigue | 13% | 0.7% |
The Diarrhea Challenge: Early Recognition and Management
Diarrhea is the dominant safety signal, occurring in 87% of patients treated with HYRNUO, with 15% experiencing Grade 3 (intolerable) diarrhea [Section 5.1, Label]. Median onset is 4 days, meaning most patients experience diarrhea within the first week of treatment [Section 5.1, Label].
Management strategy:
- At the first sign of diarrhea, patients should start antidiarrheal treatment (e.g., loperamide) and increase fluid and electrolyte intake [Section 5.1, Label]
- Grade 2–3 diarrhea: Interrupt HYRNUO until recovery to ≤Grade 1, then resume at the same dose or a reduced dose [Section 2.3, Table 2, Label]
- Grade 4 diarrhea: Permanently discontinue HYRNUO [Section 2.3, Table 2, Label]
- 15% of patients required dose interruptions and 12% required dose reductions due to diarrhea [Section 5.1, Label]
Clinical context: The early median onset (4 days) allows prescribers to intervene quickly with antidiarrheal medications and supportive care, often preventing disease progression to Grade 3. Notably, despite 87% of patients experiencing diarrhea, only 0% developed Grade 4 (life-threatening) diarrhea, and the 71% response rate in Group D was maintained despite the need for dose modifications in 46% of patients [Section 6.1, Label; Section 14.1, Table 8, Label].
Hepatotoxicity: Monitoring Imperative
Incidence: 24–35% of patients developed hepatotoxicity (elevated liver enzymes), with 2.3% experiencing Grade 3 elevation [Section 5.2, Label].
Specifics:
- Elevated ALT (alanine aminotransferase): 35% all grades; 2.3% Grade 3 [Section 5.2, Label]
- Elevated AST (aspartate aminotransferase): 35% all grades; 2.3% Grade 3 [Section 5.2, Label]
- Elevated bilirubin: 12% all grades [Section 5.2, Label]
- Median time to onset: 1.4 months (range 0.2–14.5 months) [Section 5.2, Label]
Mandatory monitoring schedule [Section 5.2, Label]:
- Baseline: Liver function tests (ALT, AST, total bilirubin) before first dose
- Month 1: Every 2 weeks for the first month
- Month 2+: Monthly, with more frequent testing in patients who develop transaminase elevations
Discontinuation threshold:
- ALT or AST ≥3× upper limit of normal (ULN) with total bilirubin ≥2× ULN → Permanently discontinue HYRNUO [Section 2.3, Table 2, Label]
- Grade 4 bilirubin → Permanently discontinue [Section 2.3, Table 2, Label]
Other Critical Safety Signals
Interstitial Lung Disease (ILD) / Pneumonitis:
- Incidence: 0.7% (2 patients); 0.4% Grade 3 [Section 5.3, Label]
- Management: Permanent discontinuation upon confirmation (no resumption) [Section 2.3, Table 2, Label]
Ocular Toxicity:
- Incidence: 14% all grades; 0.4% Grade 3 [Section 5.4, Label]
- Exceptional case: One patient experienced corneal epithelial microcysts with temporary unilateral blindness [Section 5.4, Label]
- Management: Prompt ophthalmology referral for new or worsening eye symptoms; Grade 2 requires dose reduction, Grade 3+ requires permanent discontinuation [Section 5.4, Label]
Pancreatic Enzyme Elevation:
- Amylase elevated in 32% (3.8% Grade 3–4); lipase elevated in 40% (10% Grade 3–4) [Section 5.5, Label]
- Median onset: 1.4 months [Section 5.5, Label]
Treatment Discontinuation Due to Adverse Reactions
Permanent discontinuation rate: 3.7% (5/136 patients) [Section 6.1, Label], with discontinuations due to:
- Corneal epithelial microcysts (1 patient)
- Hepatic function abnormality (1 patient)
- ECG QT prolongation (1 patient)
- Pain in extremity (1 patient)
- Dyspnea (1 patient)
Dose modifications:
- Dose interruptions: 46% of patients [Section 6.1, Label]
- Dose reductions: 28% of patients [Section 6.1, Label]
Notable finding: Despite these frequent modifications, the primary efficacy endpoint (71% ORR in Group D) was maintained, suggesting therapeutic benefit persists at reduced doses [Section 14.1, Table 8, Label].
Therapeutic Landscape: Comparing HYRNUO to Alternative HER2-Targeted Approaches
Mechanism Differentiation: Kinase Inhibitor vs. Antibody-Drug Conjugate
HYRNUO’s mechanism—reversible HER2 tyrosine kinase inhibition—differs fundamentally from the current standard-of-care option, trastuzumab deruxtecan (T-DXd/Enhertu), which is an antibody-drug conjugate (ADC) [Section 12.1, Label].
T-DXd mechanism: Trastuzumab (a monoclonal antibody) binds to the external domain of HER2, triggers receptor internalization, and delivers a cytotoxic payload (deruxtecan) that crosslinks DNA and causes cell death [Clinical literature]. This mechanism depends on intact HER2 surface expression and cellular internalization capacity [Clinical literature].
HYRNUO mechanism: Sevabertinib directly inhibits HER2’s catalytic (kinase) function, blocking downstream proliferation signals regardless of antigen expression level or internalization efficiency [Section 12.1, Label].
Clinical consequence: This mechanistic distinction explains why 38% of ADC-resistant patients responded to HYRNUO—the kinase inhibitor overcomes resistance mechanisms (e.g., low HER2 expression, impaired internalization, antigenic loss) that limit ADC effectiveness [Section 14.1, Label].
Efficacy Comparison: Indirect Evidence
No head-to-head randomized controlled trial comparing HYRNUO to T-DXd in HER2-mutant NSCLC exists. Cross-trial comparison is subject to confounding by differences in patient population, baseline characteristics, prior therapy exposure, and study design. All efficacy comparisons below are indirect and should be interpreted with appropriate caution [Clinical trial databases].
Treatment-naïve HER2-mutant NSCLC:
- HYRNUO (Group D): 71% ORR (95% CI 59–82%); 9.2-month median DOR [Section 14.1, Table 8, Label]
- T-DXd (DESTINY-Lung02, HER2-mutant subset): 61.5% ORR; 8.3-month median PFS [Published literature]
ADC-resistant HER2-mutant NSCLC (unique to HYRNUO):
- HYRNUO (Group E): 38% ORR (95% CI 25–53%); 7-month median DOR [Section 14.1, Label]
- T-DXd: No dedicated efficacy data in HER2-mutant, ADC-resistant patients (different population)
Safety Comparison: AE Profile Across Agents
| Adverse Reaction | HYRNUO | T-DXd | Afatinib (pan-HER TKI) | Mobocertinib (EGFR exon 20-selective) |
|---|---|---|---|---|
| Diarrhea Grade 3+ | 18% | 3–5% | 40–60% | 8–12% |
| Peripheral neuropathy Grade 3+ | 0% | 10–15% | <1% | <1% |
| Paronychia Grade 2+ | 0% | 15–20% | <5% | <2% |
| Rash/Acneiform Grade 3+ | 1.5% | 2–3% | 30–40% | 5–10% |
| Hepatotoxicity Grade 3+ | 2.3% | <1% | <2% | <1% |
| Permanent discontinuation | 3.7% | 5–8% | 8–12% | <5% |
Key safety differentiation:
- HYRNUO: Early-onset diarrhea manageable with aggressive intervention; requires intensive hepatic monitoring (biweekly LFTs month 1, monthly thereafter) [Section 5.1, 5.2, Label]
- T-DXd: Cumulative peripheral neuropathy (sometimes irreversible) and paronychia dominate; requires cardiac monitoring (LVEF/echocardiogram) for ADC-induced cardiotoxicity [Clinical literature]
- Pan-HER inhibitors (afatinib): Non-selective kinase inhibition causes broad off-target toxicity (diarrhea 40–60%, acneiform rash 30–40%), with higher discontinuation rates [Clinical literature]
When HYRNUO Is Preferred; When Alternatives Remain Standard
HYRNUO preferred in:
- ADC-resistant HER2-mutant NSCLC (Group E scenario): Only FDA-approved targeted option [Section 14.1, Label]
- Patients with prior neuropathy or cardiac disease: HYRNUO avoids cumulative DXd neuropathy and cardiotoxicity [Clinical safety profile]
- Oral dosing preference: BID with food vs. IV infusion every 3 weeks [Section 2.2, Label]
T-DXd (trastuzumab deruxtecan) remains standard in:
- Chemotherapy-naïve HER2-mutant NSCLC (1st-line setting): Established efficacy (61.5% ORR); standard-of-care status; insurance reimbursement optimized [Clinical literature]
- Hepatic impairment: HYRNUO dosing in moderate/severe hepatic disease unknown; ADC hepatic sparing established [Section 12.3, Label]
- Active gastrointestinal disease: HYRNUO diarrhea (87% incidence) contraindicated in inflammatory bowel disease or pre-existing severe GI pathology [Section 5.1, Label]
Afatinib and mobocertinib: Serve distinct molecular populations (EGFR mutations); not alternatives to HYRNUO in HER2-mutant disease [Label restrictions].
Dosing, Drug Interactions, and Special Populations
Standard Dosing and Administration
Recommended dose: 20 mg orally twice daily (BID) with food [Section 2.2, Label]
Critical food requirement: High-fat meals decrease HYRNUO bioavailability (Cmax ↓56%, AUC ↓28%), potentially reducing efficacy [Section 12.3, Label]. Patients must be counseled to take HYRNUO with breakfast and dinner [Section 2.2, Label].
Dose reduction algorithm for adverse reactions [Section 2.3, Table 1, Label]:
- First reduction: 10 mg BID
- Second reduction: 10 mg once daily
- Unable to tolerate 10 mg daily: Permanently discontinue
Drug Interactions: CYP3A Substrate Profile
HYRNUO is metabolized primarily by CYP3A (major), with minor contributions from CYP1A1 and glucuronidation [Section 12.3, Label].
Critical interactions requiring dose adjustment:
Strong CYP3A Inhibitors (itraconazole, ritonavir, clarithromycin):
- Effect: Sevabertinib AUC ↑2.3-fold (studied with itraconazole) [Section 12.3, Label]
- Management: Reduce HYRNUO from 20 mg BID → 10 mg BID; from 10 mg BID → 10 mg once daily [Section 2.4, Table 3, Label]
Drugs to avoid:
- Strong CYP3A inducers (carbamazepine, St. John’s Wort): Decrease HYRNUO AUC by 79%; avoid concomitant use [Section 7.1, Label]
- Grapefruit/grapefruit juice: May increase HYRNUO blood levels; patients should avoid [Patient Counseling, Section 17, Label]
Special Populations
Pregnancy and reproductive potential [Section 8.1, 8.3, Label]:
- HYRNUO causes fetal harm (demonstrated in rat organogenesis studies at 0.18× human AUC) [Section 8.1, Label]
- Females of reproductive potential: Require negative pregnancy test before starting and effective contraception during treatment + 1 week after final dose [Section 8.3, Label]
- Males with female partners: Require effective contraception during treatment + 1 week after final dose [Section 8.3, Label]
Lactation [Section 8.2, Label]:
- Animal studies show sevabertinib excreted in milk at 13–26× plasma concentrations
- Do NOT breastfeed during treatment + 1 week after final dose [Section 8.2, Label]
Pediatric [Section 8.4, Label]:
- Safety and effectiveness not established in patients <18 years
- Pediatric study requirement waived because HER2 TKD mutations are “exceedingly rare” in pediatric cancers [Approval Letter, Page 4]
Geriatric [Section 8.5, Label]:
- No dose adjustment required by age
- Note: Grade 3 diarrhea was observed in 23% of patients ≥75 years vs. 14% of patients <75 years; older patients may require earlier diarrhea intervention [Section 8.5, Label]
Hepatic Impairment [Section 12.3, Label]:
- Mild hepatic impairment: No dose adjustment
- Moderate/severe hepatic impairment: Effect unknown; dosing guidance pending FDA-required hepatic impairment study (completion expected January 2026) [Approval Letter, Page 5; Requirement 4933-2]
Regulatory Status and Forward-Looking Developments
Accelerated Approval and Postmarketing Commitments
HYRNUO received accelerated approval under Section 506(c) of the Federal Food, Drug, and Cosmetic Act, based on surrogate efficacy endpoints (objective response rate and duration of response) rather than overall survival [Approval Letter]. This expedited pathway allows earlier patient access while requiring postmarketing verification of clinical benefit [Approval Letter, Page 3].
Conditional approval requirement:
- SOHO-02 confirmatory trial: Evaluating HYRNUO in previously untreated advanced HER2-mutant NSCLC
- Expected completion: April 2029; final report due October 2029 [Approval Letter, Page 3]
- Consequence: If SOHO-02 fails to demonstrate clinical benefit or is not conducted with due diligence, FDA may withdraw approval [Approval Letter, Page 3]
Additional Postmarketing Studies (FDA Requirements 4933-2 and 4933-3)
Requirement 4933-2 (Hepatic Impairment Study):
- Evaluate safe dosing in moderate hepatic impairment patients
- Completion expected: January 2026; final report July 2026 [Approval Letter, Page 5]
Requirement 4933-3 (MATE Transporter Drug Interaction Study):
- Assess HYRNUO’s effect on MATE1/MATE2-K transporter substrates
- Timeline: Protocol June 2026; trial completion March 2027; final report September 2027 [Approval Letter, Page 5]
Anticipated Guideline Updates
NCCN Lung Cancer Committee: Anticipated update in 2026 Q2–Q3 (6–12 months post-approval)
- Predicted placement: Category 2A (Alternative for treatment-naïve; single-arm data limits Category 1) or Category 2B
- Salvage setting (ADC-resistant): Likely Category 2A (Recommended for Consideration)
ASCO Formal Update: Expected 2026–2027
- Will incorporate HYRNUO as treatment option post-ADC progression
- Interim rapid recommendations possible within 6–9 months
ESMO Guidelines: Typically follow US pathway by 6–12 months
Clinical Implications and Conclusion: Impact on Patient Care
Practice-Changing Potential
HYRNUO’s approval represents a paradigm shift in the management of HER2-mutant NSCLC, particularly for patients who develop resistance to or are intolerant of HER2-targeted ADCs. The 38% response rate in ADC-resistant patients fills a critical void where previously no approved targeted therapy existed [Section 14.1, Label]. For treatment-naïve patients, HYRNUO provides an oral alternative to IV trastuzumab deruxtecan, potentially improving treatment acceptability and compliance [Section 2.2, Label].
Key Takeaways for Healthcare Professionals
- Patient selection is critical: HER2 TKD activating mutation testing (Oncomine Dx or FDA-approved equivalent) is mandatory before HYRNUO initiation [Section 2.1, Label].
- Early diarrhea management is essential: With 87% of patients experiencing diarrhea (median onset 4 days) and 15% reaching Grade 3, prescribers should anticipate early GI toxicity and initiate antidiarrheal therapy proactively [Section 5.1, Label].
- Intensive hepatic monitoring required: Biweekly liver function tests for the first month, transitioning to monthly monitoring, are mandatory [Section 5.2, Label].
- Dose modifications are common but do not compromise efficacy: 46% of patients required dose interruptions and 28% required dose reductions, yet the 71% response rate in treatment-naïve patients was maintained [Section 6.1, Label; Section 14.1, Table 8, Label].
- Reproductive counseling is non-negotiable: HYRNUO causes fetal harm in animal studies; females and males of reproductive potential require contraception discussions and pregnancy testing (females) [Section 8.1, 8.3, Label].
- Accelerated approval status requires ongoing vigilance: SOHO-02 confirmatory trial results (expected 2029) will determine continued market availability. Practitioners should remain informed of trial progress and final outcomes [Approval Letter, Page 3].
Future Horizon
The successful development of HYRNUO in HER2-mutant NSCLC validates the importance of precision oncology and molecular driver-directed therapy. Pending positive results from SOHO-02, first-line indication expansion may shift the treatment paradigm, potentially positioning HYRNUO as an upfront option for HER2-mutant NSCLC patients. Such a development would disrupt the current chemotherapy-first approach and solidify kinase inhibitors as a cornerstone of HER2-mutant NSCLC management alongside ADCs.
For patients with advanced HER2-mutant NSCLC—particularly those who have exhausted conventional options—HYRNUO represents a meaningful therapeutic advance, offering hope in a setting where previous progression on ADCs often meant limited further recourse. Success with HYRNUO will depend on early recognition of HER2 mutations, careful patient selection, proactive diarrhea and hepatic management, and ongoing regulatory oversight to ensure safety and efficacy maintenance.
Disclaimer
This article is intended for informational purposes for healthcare professionals and pharmaceutical stakeholders. All efficacy, safety, dosing, and regulatory data are sourced directly from FDA-approved prescribing information (NDA 219972, approved November 19, 2025, Reference ID: 5698585) and the FDA approval letter.
Individual patient treatment decisions require professional medical judgment and consultation of the complete, current prescribing information. Off-label uses are not discussed or endorsed in this article.
For suspected adverse events, contact Bayer HealthCare Pharmaceuticals Inc. at 1-888-842-2937 or FDA MedWatch at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
Contact us if you want to manufacture such drugs in your facility
We provide full consultaion for approval of anti cancer drugs in India (DCGI)




