Avutometinib-Defactinib Co-Pack: A Breakthrough for KRAS+ Low-Grade Serous Ovarian Cancer
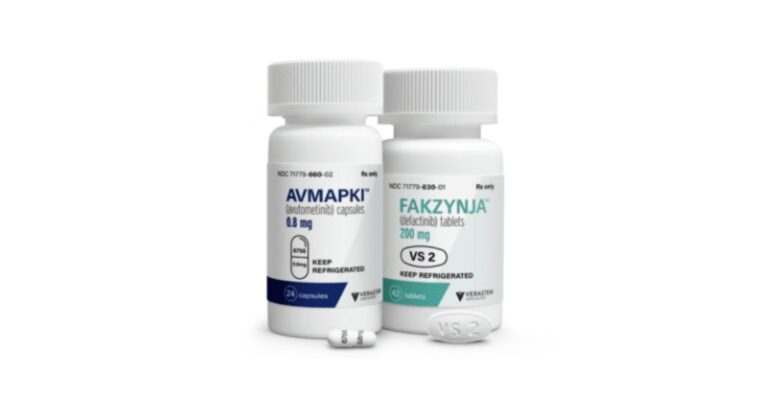
On May 08, 2025 , the FDA granted accelerated approval to a novel oral therapy for a rare form of ovarian cancer. Avutometinib (a “RAF-MEK clamp” inhibitor) and Defactinib (a FAK inhibitor) are now co-packaged as Avmapki Fakzynja Co-Pack and…