Prolonged release vs sustained release tablets sit under the broader “modified release” family but differ in their pharmacokinetic goals, release kinetics, and clinical use cases. Understanding these nuances is essential for formulation scientists, regulatory teams, and plant owners when designing products, planning bioequivalence, or drafting quality and CMC documentation.
Modified release: core concepts
Modified release (MR) dosage forms deliberately change the rate, site, or timing of drug release compared with an immediate release (IR) reference. Regulatory guidelines from EMA, FDA, and other agencies describe MR as a broad category that includes extended, delayed, sustained, controlled, prolonged, and pulsatile systems.
In practice, oral MR tablets and capsules aim to:
- Maintain therapeutic plasma levels over longer intervals
- Reduce dosing frequency and improve adherence
- Minimize peaks and troughs that drive side effects or loss of efficacy
Within this umbrella, sustained release (SR) and prolonged release (PR) are two related but distinct approaches.
What is sustained release (SR)?
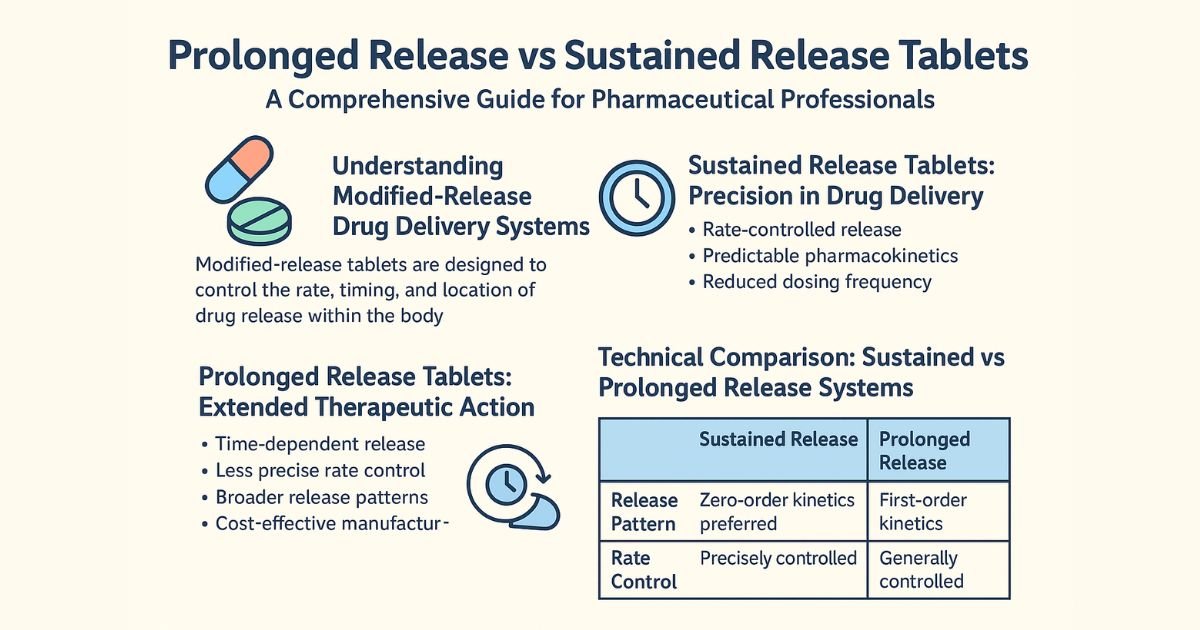
Sustained release formulations are designed to release the active ingredient gradually over an extended period to maintain drug concentration within the therapeutic window for a defined dosing interval. By smoothing the input rate, SR products aim to approximate a near steady-state plasma level after each dose, reducing fluctuations relative to an equivalent IR regimen.
Key points for sustained release tablets:
- Purpose: Maintain relatively constant plasma concentration over a specified period (e.g., 8–12 hours), often approaching zero‑order or near zero‑order input.
- Kinetics: More controlled and uniform release rate, often designed to meet predictable in vitro–in vivo correlation (IVIVC) targets.
- Clinical aim: Continuous therapeutic effect with reduced dosing frequency and typically fewer peak‑related adverse events.
SR technologies include hydrophilic and hydrophobic matrix tablets, coated multiparticulates, osmotic pump systems, and reservoir-type coatings.
What is prolonged release (PR)?
Prolonged release systems extend the duration of drug action beyond that achieved with conventional IR products, but do not necessarily maintain a constant plasma concentration. The primary objective is to keep levels above the minimum effective concentration for longer, often allowing once‑ or twice‑daily dosing where IR would require more frequent administration.
Key points for prolonged release tablets:
- Purpose: Extend the time the drug remains at clinically effective levels, without strictly targeting a flat plasma profile.
- Kinetics: Gradual, often first‑order‑like release, with plasma concentrations that rise slower than IR and decline more slowly, but still show more fluctuation than an ideal SR profile.
- Clinical aim: Longer action and better convenience, accepting some variation in concentration as long as it stays within acceptable efficacy and safety margins.
Prolonged release can be achieved with slower‑dissolving matrices, modified disintegration properties, or coating strategies that slow but do not fully control the release rate.
Extended release vs sustained release vs prolonged release
“Extended release” is often used as an umbrella term in both regulatory and marketing language, under which sustained and prolonged release systems sit as subtypes. However, many companies and formularies use ER and SR interchangeably, which can obscure their different design intent.
Common distinctions:
- Extended release (ER): Any formulation that extends the release or action of the drug versus IR, covering both sustained and prolonged systems.
- Sustained release (SR): Extended release with a design focus on maintaining relatively constant plasma levels for a defined interval.
- Prolonged release (PR): Extended release focused on lengthening duration above the minimum effective concentration rather than minimizing fluctuation.
From a regulatory and QbD perspective, clearly stating whether a product claims “controlled/sustained” versus “prolonged” release helps define release specifications, dissolution profile targets, and bioequivalence study design.
Prolonged release vs sustained release: key differences
The table below summarizes the practical, kinetic, and formulation differences that matter for development and regulatory submissions.
Tablet profile differences
| Parameter | Sustained Release (SR) | Prolonged Release (PR) |
|---|---|---|
| Primary objective | Maintain near‑constant plasma concentration over a set dosing interval | Extend duration of effect beyond IR while remaining above minimum effective concentration |
| Release rate | Controlled and often close to zero‑order or pre‑defined profile | Gradual, typically first‑order‑like, may not be uniform |
| Plasma concentration pattern | Flatter profile with reduced peaks and troughs | Slower rise and slower fall vs IR, but more fluctuation than SR |
| Dosing frequency | Often once or twice daily for drugs that otherwise require multiple daily IR doses | Often reduces dose frequency vs IR, but not always as much as optimized SR |
| Typical technologies | Complex matrices, osmotic pumps, multi‑layer tablets, multiparticulates | Slower‑dissolving matrices, coatings delaying disintegration or diffusion |
| Risk of dose dumping | High regulatory scrutiny; robust controls and dissolution testing required | Still scrutinized but often with less aggressive “flat profile” targets |
| Clinical messaging | “Maintains steady control” or “smooth 24‑hour coverage” | “Long‑lasting relief” or “extended action” |
Data points in this table reflect descriptions from pharmaceutics references and industry guidance discussing sustained vs prolonged release characteristics.
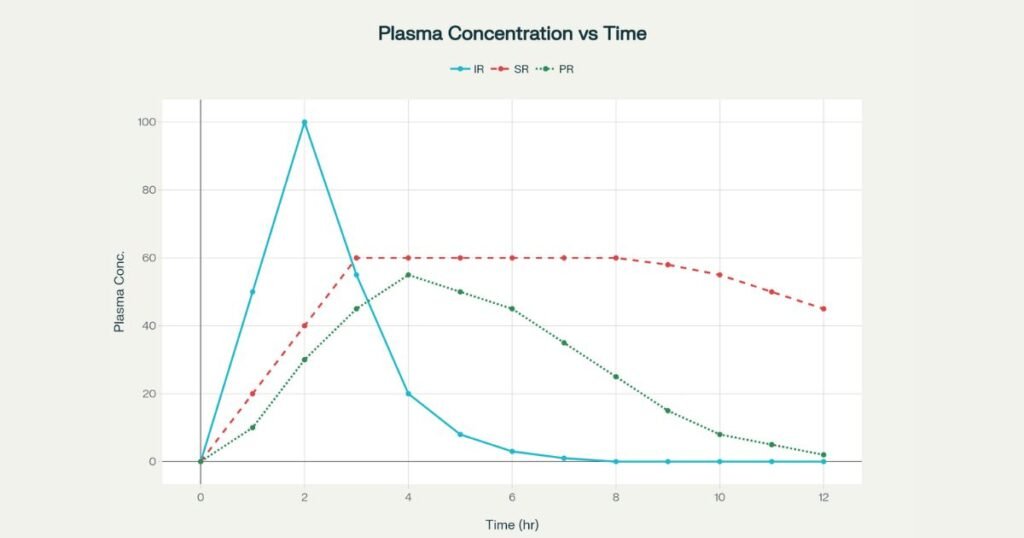
Pharmacokinetics: concentration–time behavior
In an IR tablet, most of the dose is released rapidly in the upper GI tract, leading to a sharp peak (Cmax) followed by a rapid decline, often creating subtherapeutic troughs between doses. Sustained and prolonged release curves reshape this profile by modifying input rate relative to elimination.
Typical PK patterns:
- IR: High Cmax, short Tmax, marked fluctuations; may cross toxicity thresholds or drop below efficacy threshold multiple times per day.
- SR: Slower rise, lower Cmax, extended plateau within the therapeutic window, with a smoother decline near the end of the dosing interval.
- PR: Intermediate rise and decline; peak lower than IR but higher and less flat than SR, with extended time above minimum effective concentration.
Regulators increasingly emphasize parameters like Cτ (concentration at end of dosing interval) and partial AUC to compare MR and IR profiles and to support claims of reduced fluctuation or extended coverage.
Regulatory views and terminology
Major regulatory bodies do not always draw a sharp line between “sustained” and “prolonged” in legal definitions, but they are precise about the broader concept of modified/extended release.
- EMA guideline on pharmacokinetic and clinical evaluation of modified release dosage forms outlines study designs, PK endpoints, and clinical rationale required for MR formulations (oral, parenteral, transdermal).
- FDA SUPAC‑MR guidance addresses post‑approval changes for MR solid orals, emphasizing identification of release‑controlling excipients and control of dissolution profiles to maintain performance.
Implications for SR vs PR positioning:
- If a label or promotional material claims “controlled/sustained release” with reduced fluctuation, regulators expect data demonstrating comparable or smoother PK versus IR at steady state.
- For products framed more generally as “prolonged” or “extended‑action,” the burden is typically to show acceptable exposure, duration, and safety versus IR without necessarily proving near‑constant levels.
For markets following ICH and EMA guidance (including many WHO‑GMP/USFDA‑oriented facilities in India), dossier terminology should match actual PK behavior and dissolution data to avoid misalignment during review.
Formulation strategies for SR vs PR tablets
Sustained release design focus
SR tablets require tighter engineering to approach a constant release rate across variable GI conditions. Typical approaches include:
- Hydrophilic matrix tablets (e.g., HPMC) designed for controlled swelling and erosion
- Hydrophobic matrices or waxy systems controlling diffusion
- Multiparticulate systems (pellets, mini‑tablets) with controlled coating thicknesses
- Osmotic pump tablets with semi‑permeable membranes and laser‑drilled orifices
These systems must demonstrate robust, reproducible dissolution under multiple media and agitation conditions, especially when linked to IVIVC or biowaivers.
Prolonged release design focus
PR tablets often use simpler or lower‑intensity control mechanisms, prioritizing extended release over perfect flatness. Examples include:
- Matrices with slower‑dissolving excipients that delay complete release
- Coatings that slow disintegration or diffusion but are less tightly tuned than SR
- Modified particle size distribution or salt forms that inherently slow dissolution
These products can be attractive in cost‑sensitive markets or for APIs where precise control is less critical, provided therapeutic levels are maintained safely.
Clinical and patient‑centric considerations
From a clinical perspective, both SR and PR aim to improve adherence and outcomes compared with IR dosing. However, choice of strategy depends on disease, pharmacology, and risk profile of the API.
Common benefits of SR and PR:
- Reduced dosing frequency (once or twice daily vs multiple IR doses) improving adherence
- Smoother symptom control and fewer “wear‑off” episodes, particularly with SR where fluctuation is lower
- Potential reduction in peak‑related adverse events, especially for drugs with narrow safety margins and concentration‑related side effects
Trade‑offs:
- More complex manufacturing and higher development cost compared with IR forms
- Risk of dose dumping if coatings or matrices fail, especially with strong alcohol co‑ingestion or GI motility changes
- Challenges in dose individualization if tablets cannot be split or crushed safely
For chronic conditions (hypertension, diabetes, chronic pain), SR can be particularly valuable where tight control and minimal fluctuation are desired. For symptom‑driven conditions where convenience is the main goal, PR may be acceptable if total exposure and safety remain appropriate.
Difference between sustained release and extended release tablets
Many formularies and patient‑facing resources equate “SR” with “ER,” but some technical distinctions are helpful.
- Extended release (ER) describes any formulation that releases a drug over a longer period than IR, sometimes without specifying the exact profile.
- Sustained release (SR) is a subset of ER that specifically targets a prolonged, relatively constant exposure to minimize fluctuations over the dosing interval.
In practice:
- An ER label might apply to a product with a gradual decline and more pronounced peak–trough difference (PR‑like behavior).
- An SR label is better reserved for profiles with controlled, near‑steady input and demonstrated reduction in fluctuation indices relative to IR.
When developing content for professionals, it is advisable to use “modified/extended release” as the overarching term, and then clarify whether a particular product is functionally SR or PR based on its PK data.
Development and validation: what changes between SR and PR?
Pre‑formulation and biopharmaceutics
Pre‑formulation studies for both SR and PR assess:
- API solubility and permeability (BCS class)
- Half‑life, therapeutic window, and target exposure profile
- Food effect and GI transit behavior
For SR:
- APIs with short half‑life and relatively narrow therapeutic windows are often preferred, because controlled input can meaningfully reduce fluctuations.
- Strong emphasis on achieving a predefined input rate matching the desired plasma plateau.
For PR:
- Half‑life and therapeutic window still matter, but the emphasis is on ensuring extended time above minimum effective concentration with acceptable safety margins.
In vitro–in vivo correlation and dissolution
Regulators encourage robust dissolution methods that differentiate SR and PR products, especially when changes are made post‑approval.
- SR formulations often target tighter IVIVC, enabling predictive bioequivalence assessments for certain changes.
- PR formulations may allow somewhat broader dissolution windows as long as PK exposure and clinical performance remain within justified ranges.
SUPAC‑MR guidance defines levels of post‑approval changes (components, process, equipment, site, batch size) and corresponding dissolution and bioequivalence requirements to maintain performance.
When to choose prolonged vs sustained release for a new product
Formulation and business decisions often weigh technical feasibility, regulatory expectations, and market positioning.
Situations favoring sustained release (SR):
- API with short half‑life and narrow safety margin
- Indications where stable control is crucial (e.g., blood pressure, seizure control)
- High‑value markets where premium pricing is justified and regulators expect robust PK claims
Situations favoring prolonged release (PR):
- API with relatively wider therapeutic window where some fluctuation is acceptable
- Markets prioritizing convenience and cost over tight PK control
- Lifecycle‑management products where modest development investment can still deliver a differentiating once‑ or twice‑daily regimen
Clear internal language (e.g., “PR convenience product” vs “SR control product”) helps align R&D, regulatory, and marketing teams.
How Laafon.com can help you?
- For plant owners and formulation teams planning SR or PR products for USFDA/WHO‑GMP markets, consider a dedicated consultation on MR facility readiness, QbD documentation, and scale‑up strategy. Get Help from us:
FAQ
How should bioequivalence studies differ between SR and PR generics?
Both require demonstration of comparable exposure (AUC) and maximum concentration (Cmax), but SR generics may also need to match fluctuation indices, partial AUCs, and concentration at the end of the dosing interval. For PR, studies primarily need to ensure similar exposure and duration above therapeutic thresholds without unacceptable peaks or troughs.
Are there special manufacturing controls for MR tablets in USFDA/WHO‑GMP plants?
Yes. MR tablets require tight control of release‑controlling excipients, coating processes, and critical process parameters, with validated dissolution methods that are stability‑indicating and discriminatory. Plants must document these controls within the pharmaceutical quality system and demonstrate consistency during scale‑up and routine production in line with SUPAC‑MR and GMP expectations.
References:
- European Medicines Agency (EMA). Guideline on the pharmacokinetic and clinical evaluation of modified release dosage forms. EMA/CHMP/EWP/280/96 Rev1. 2019.
https://www.ema.europa.eu/en/pharmacokinetic-clinical-evaluation-modified-release-dosage-forms-scientific-guideline - European Medicines Agency (EMA). Guideline on quality of oral modified release products.
https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-quality-oral-modified-release-products_en.pdf - U.S. Food and Drug Administration (FDA). SUPAC‑MR: Modified Release Solid Oral Dosage Forms: Scale‑Up and Postapproval Changes – Chemistry, Manufacturing, and Controls; In Vitro Dissolution Testing and In Vivo Bioequivalence Documentation.
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/supac-mr-modified-release-solid-oral-dosage-forms-scale-and-postapproval-changes - U.S. Food and Drug Administration (FDA). SUPAC‑MR: Modified Release Solid Oral Dosage Forms – Scale‑Up and Postapproval Changes (full PDF).
https://www.fda.gov/media/70956/download - Therapeutic Goods Administration (TGA), Australia. Guideline on the pharmacokinetic and clinical evaluation of modified release dosage forms (International Scientific Guideline adopted in Australia).
https://www.tga.gov.au/resources/resources/international-scientific-guidelines-adopted-australia/guideline-pharmacokinetic-and-clinical-evaluation-modified-release-dosage-forms - ScienceDirect (Elsevier). Sustained Drug Release – an overview.
https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/sustained-drug-release - ScienceDirect (Elsevier). Sustained Drug Release – medicine and dentistry topic page.
https://www.sciencedirect.com/topics/medicine-and-dentistry/sustained-drug-release - Khan R, et al. Formulation and evaluation of sustained release matrix tablets of rabeprazole. Available via PubMed Central (PMC).
https://pmc.ncbi.nlm.nih.gov/articles/PMC4097931/ - Research Journal of Pharmacy and Technology. Sustained Release Products: A Review on Formulation and Evaluation.
https://rjptonline.org/HTMLPaper.aspx?Journal=Research+Journal+of+Pharmacy+and+Technology%3BPID%3D2013-6-12-13 - International Journal of Pharmaceutical Research and Applications. Sustained Release Drug Delivery System: A Review.
https://ijprajournal.com/issue_dcp/Sustained%20Release%20Drug%20Delivery%20System%20%20A%20Review.pdf - European Medicines Agency (EMA). Guideline on quality of oral modified release products – statement on scoring and manipulation of prolonged release tablets.
https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-quality-oral-modified-release-products_en.pdf - Accessible Medicines. Scale Up and Post Approval Changes (SUPAC) Guidance – GRx+Biosims 2024 presentation.
https://accessiblemeds.org/wp-content/uploads/2024/12/GRxBiosims-2024-PPT-David-Awotwe-Otoo.pdf - Specac Technical Article. How Sustained‑Release Drug Release Systems Work.
https://specac.com/xrf-applications/developing-sustained-release-pharmaceuticals-sample-preparation/ - The Journal of Clinical Psychiatry. Sustained‑Release, Extended‑Release, and Other Time‑Release Formulations: A Review of Neuropsychiatric Uses.
https://www.psychiatrist.com/jcp/sustained-release-extended-release-release-formulations/ - FDA (related context). SUPAC‑IR: Immediate‑Release Solid Oral Dosage Forms: Scale‑Up and Postapproval Changes.
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/supac-ir-immediate-release-solid-oral-dosage-forms-scale-and-postapproval-changes