Are you considering a strategic move into the thriving pharmaceutical sector? The global pharmaceutical market is booming, projected to hit an astounding $1.6 trillion by 2028, driven by a robust 6.5% Compound Annual Growth Rate (CAGR). This presents a monumental opportunity for aspiring entrepreneurs and established businesses alike. However, embarking on a pharmaceutical manufacturing venture, with investments typically ranging from $2 million to over $50 million depending on scale and product focus, demands a meticulous understanding of every single cost component.
At Laafon, we understand the complexities involved. This comprehensive guide is designed to be your trusted resource, breaking down every cost, sharing regional comparisons within India, and providing actionable strategies to optimize your budget. Ultimately, we’ll help you navigate the intricate journey of setting up your pharmaceutical manufacturing plant with confidence.
GET ACTUAL COST OF PLANT BY CALCULATOR
India’s Pharmaceutical Sector: A Strategic Overview
India stands as a global powerhouse in the pharmaceutical industry, often referred to as the “Pharmacy of the World.” This leadership is underscored by impressive growth figures: according to the Ministry of Commerce and Industry, the pharma sector witnessed a 103% growth between 2013-14 and 2021-22, with absolute figures soaring from INR 90,415 Crores to INR 1,83,422 Crores. This remarkable expansion highlights the sector’s resilience and potential for a continued upward trajectory.

Why India is Your Strategic Advantage
Several factors give India a significant edge in the global pharmaceutical landscape:
- Availability of a Large Skilled Workforce: India boasts a vast and continuously growing pool of highly qualified scientists, pharmacists, engineers, and skilled technicians. This readily available talent reduces recruitment costs and ensures operational efficiency—a key advantage over many other manufacturing hubs.
- Cost-Effectiveness: Manufacturing costs in India are significantly lower compared to Western nations, making Indian pharmaceutical products highly competitive in the global market. This cost advantage extends to labor, raw materials, and operational overheads.
- Global Demand: Indian pharmaceutical generic products are in high demand globally, with countries like the US and the UK being major importers. India’s ability to produce high-quality, affordable generics has cemented its position as a reliable global supplier.
- Government Support & Incentives: The Indian government actively promotes domestic pharmaceutical manufacturing through various initiatives and policies. Programs like the Production Linked Incentive (PLI) schemes offer substantial financial incentives to boost local production and attract investments, further enhancing the sector’s attractiveness. You can learn more about specific PLI schemes on the official Ministry of Pharmaceuticals website.
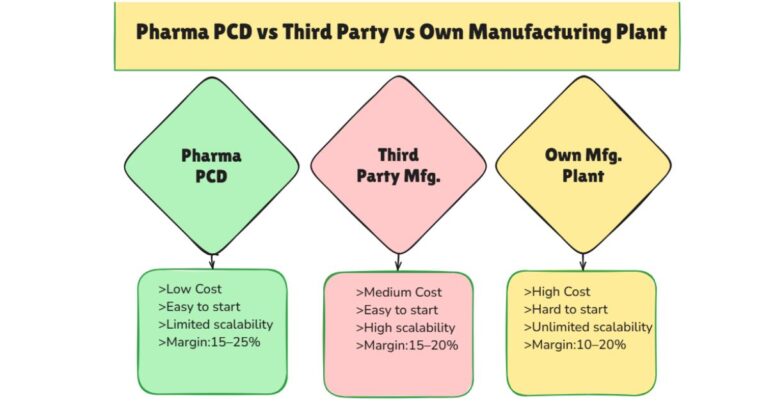
Why Establish Your Own Pharma Plant?
For business owners eyeing this thriving sector, establishing your own manufacturing plant offers unparalleled advantages. It grants you greater control over critical aspects like production processes, stringent quality assurance, and dynamic pricing strategies. This direct involvement can significantly mitigate the common challenges faced by the pharmaceutical marketing industry, such as unpredictable price fluctuations and reliance on external manufacturing partners.
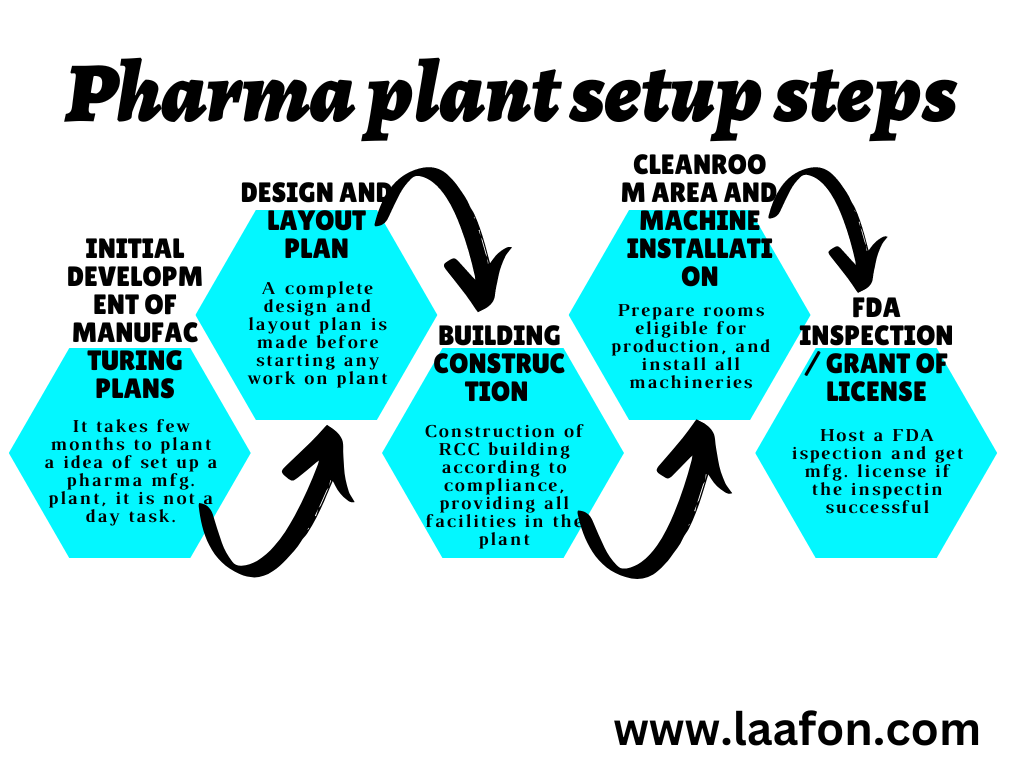
Roadmap to Setting Up Your Pharma Manufacturing Unit
Establishing a pharmaceutical manufacturing plant is a multi-phase project requiring meticulous planning, significant capital, and rigorous execution. Here’s a comprehensive roadmap outlining the critical stages:
Phase 1: Planning & Feasibility Study (1-2 months)
This foundational phase is crucial, as it meticulously sets the direction for your entire project. Investing adequately in this stage, often with expert consultancy, can prevent costly errors and delays down the line.
- Market Research & Product Selection: Beyond identifying general market demand, this involves deep dives into specific therapeutic areas (e.g., cardiology, oncology, dermatology) and dosage forms (e.g., oral solids like tablets and capsules, liquids like syrups and suspensions, injectables, ointments, or even hormonals). Your product portfolio will directly dictate the type of machinery, cleanroom classifications, and regulatory pathways required, significantly impacting your overall expenditure.
- Business Plan Development (DPR): Creating a Detailed Project Report (DPR) is paramount. This document serves as your project’s blueprint, meticulously outlining:
- Your overarching vision.
- A comprehensive market analysis, including target audience, competitive landscape, and pricing strategy.
- A detailed operational plan, covering production capacity, supply chain management, and robust quality management systems.
- Reliable financial projections, encompassing initial investment, operating costs, revenue forecasts, and break-even analysis. A well-crafted DPR is truly indispensable for attracting investors and securing necessary financing.
- Funding & Financing Strategy: Explore various funding avenues such as traditional bank loans, venture capital, private equity, or government subsidies and grants specifically designed for the pharmaceutical sector. Understanding the requirements and timelines for each option, along with a clear Return on Investment (ROI) projection, is crucial for your financial success.
Phase 2: Land Acquisition & Legal Formalities (2-3 months)
With meticulous planning complete, the focus shifts to establishing the physical foundation of your pharmaceutical plant.
- Site Selection: Choosing the right location is strategic. Factors include proximity to raw material suppliers, accessibility to major transportation hubs (roads, ports, airports), availability of skilled labor, reliable utility infrastructure (power, water), and favorable local regulatory bodies. Locations within industrial parks or designated pharma zones often simplify approvals and offer access to shared resources or a concentrated talent pool.
- Land Acquisition: This involves purchasing or leasing land that is appropriately zoned for industrial use and meets the required area for your facility, including ample space for future expansion plans.
- Legal Formalities: Meticulously complete all necessary land registrations, property transfers, and Environmental Impact Assessments (EIAs) as mandated by local laws.
Phase 3: Initial Regulatory Approvals (Pre-Construction) (3-6 months)
Before breaking ground, critical environmental and building permits are needed. This phase is non-negotiable for compliance.
- Environmental Clearances: Obtain essential No Objection Certificates (NOCs) from the State Pollution Control Board, confirming your proposed plant’s environmental compliance. This often involves submitting a detailed environmental management plan.
- Zoning & Building Permits: Secure all necessary approvals from local municipal corporations or industrial development authorities for the construction of your facility, ensuring strict adherence to local building codes and zoning regulations. Our experience shows that a pre-construction regulatory checklist is vital to avoid delays here.
Phase 4: Construction & Infrastructure Development (6-8 months)
This is the physical construction phase, with a strong emphasis on specialized pharmaceutical requirements.
- Facility Design & Layout: Develop highly detailed architectural, structural, and utility drawings. This includes meticulous planning for HVAC systems (critical for maintaining precise environmental conditions), electrical layouts, plumbing, and robust fire safety systems. The design must incorporate unidirectional material and personnel flow to prevent cross-contamination.
- Construction: Build the manufacturing facility, which comprises specialized cleanroom areas (classified according to ISO standards, e.g., ISO Class 8 for general production, ISO Class 7 for aseptic filling), dedicated warehouses for raw materials and finished goods, administrative blocks, quality control laboratories, and utility buildings. Critically, construction materials must be non-shedding, easy to clean, and resistant to chemicals.
Phase 5: Machinery Installation & Quality Control Setup (2-3 months)
This phase focuses on equipping your facility, transforming the physical structure into a functional pharmaceutical manufacturing unit.
- Procurement & Installation: This involves purchasing and installing all primary production machinery (e.g., tablet presses, liquid filling lines), essential utility equipment (e.g., boilers, air compressors), and sophisticated quality control laboratory instruments.
- Validation & Calibration: This is a critical step where all installed equipment is rigorously validated to ensure it performs as intended and consistently produces products meeting specifications. This includes Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Regular calibration of instruments is also mandatory for accurate measurements.
Phase 6: Workforce Hiring & Training (1-2 months)
Building the human capital is as crucial as the physical infrastructure for your plant’s success.
- Recruitment: Hire key personnel who are experienced and qualified. This includes a Production Manager, Quality Control Manager, Quality Assurance Manager, skilled operators, maintenance technicians, and administrative support staff.
- Training: Provide comprehensive and ongoing training programs covering Good Manufacturing Practices (GMP), Standard Operating Procedures (SOPs) for all processes, equipment operation, safety protocols, and quality management systems. Emphasize continuous professional development to retain top talent.
Phase 7: Final Regulatory Inspections & Certifications (3-6 months for initial, 12+ months for WHO-GMP)
This is the final hurdle before commercial production can commence, ensuring full legal and quality compliance.
- Manufacturing License: Obtain the primary license from the State Drug Control Department, allowing you to legally manufacture specific pharmaceutical products.
- GMP (Good Manufacturing Practices) Certification: This is a fundamental quality system ensuring products are consistently produced and controlled according to quality standards. It covers all aspects of manufacturing, from starting materials, premises, and equipment to the training and personal hygiene of staff. This is issued by the State Drug Control Department.
- WHO-GMP Certification: For companies intending to export products to many international markets or participate in government tenders, obtaining WHO-GMP certification is essential. This international standard often requires more stringent audits and can take a further 12 months post-domestic GMP certification. Navigating state-specific regulatory nuances is key here.
- Other Essential Licenses: Drug sales license, consent from the Pollution Control Board, factory license, and a No Objection Certificate (NOC) from the Fire Safety Department are also mandatory.
- Approval Process Costs: These include application fees, inspection fees levied by regulatory bodies, and potentially significant consultant fees for dossier preparation, facility design review, and compliance guidance. Adherence to ICH guidelines (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use) is often crucial for global market access.
Phase 8: Trial Production & Commercial Launch (1-2 months)
The culmination of your efforts—preparing for and initiating full-scale production.
- Trial Runs: Conduct several trial production batches to fine-tune manufacturing processes, identify bottlenecks, and ensure consistent product quality. This is a crucial learning phase.
- Quality Testing: Rigorous testing of these trial batches in the QC lab to confirm they meet all specified quality parameters before full-scale production.
- Commercial Production: Once all quality checks are cleared and licenses are in place, commence full-scale manufacturing and distribution.
Detailed Cost Components: Breaking Down Your Pharma Plant Investment
The investment required for a pharmaceutical manufacturing plant can be broadly categorized into the following components, with significant variations based on scale, location, and product type:
1. Land & Infrastructure Costs
These costs are highly dependent on location, required area, and the level of sophistication of the facility, especially concerning cleanroom requirements.
- Land Acquisition:
- Cost: Varies significantly by region and whether it’s an urban industrial zone (with better infrastructure but higher costs) or a rural area (lower land cost but potentially less developed infrastructure). Factors like proximity to highways, ports, raw material suppliers, and research hubs/talent pools also influence price.
- Example Land Prices (INR Per Sq.Ft – indicative):
| Region | Urban Area (Per Sq.Ft) | Rural Area (Per Sq.Ft) |
| Hyderabad, India | 8000 | 3500 |
| Uttarakhand, India | 8000 | 4500 |
| Haryana, India | 8000 | 4500 |
- Construction & Civil Works:
- Cost: INR 1.5 – 2 crores for a 15,000 sq. ft. facility. This includes the structural framework, roofing, and foundational elements.
- Key Specialized Elements for Pharma:
- Specialized Flooring: Epoxy resin (approx. INR 90/sq ft) is commonly used for its seamless, non-porous, chemical-resistant, and easy-to-clean properties, crucial for contamination control and hygiene. Anti-static flooring may be required in certain areas, particularly where sensitive electronic equipment is used.
- Cleanroom Wall Panels & Ceilings: These are typically made of modular, pre-fabricated panels (e.g., PUF/EPS insulated panels) that are non-shedding, smooth, and easily sanitized. They ensure airtight seals and prevent particle generation, critical for maintaining air purity.
- Doors & Pass Boxes: Interlocked doors and pass boxes (static or dynamic) are essential for maintaining differential pressures between cleanroom grades and preventing cross-contamination during material transfer.
- HVAC (Heating, Ventilation, and Air Conditioning) Systems:
- Cost: INR 1 cr. to 1.5 cr.
- Importance: HVAC systems are the backbone of cleanroom environments. They are critical for maintaining precise temperature, humidity, and air purity levels. This involves sophisticated Air Handling Units (AHUs), HEPA (High-Efficiency Particulate Air) filters, and extensive ducting. Different areas of the plant require specific ISO classifications (e.g., ISO Class 8 for general production, ISO Class 7 for aseptic filling areas, ISO Class 5 for sterile filling zones), each with specific air changes per hour and filtration requirements, directly impacting HVAC system complexity and cost.
- Water Purification Systems:
- Cost: INR 50 lakhs – 1 crore.
- Types: Pharmaceutical manufacturing requires high-purity water.
- Purified Water (PW) plants: Typically use Reverse Osmosis (RO) and deionization for general use like cleaning, granulation, and non-sterile product formulation.
- Water for Injection (WFI) plants: Essential for sterile products and injectables, WFI systems produce water of much higher purity, often through distillation or advanced membrane technologies, which are significantly more expensive to install and maintain due to their complexity and validation requirements.
- Electrical & Utility Infrastructure:
- Cost: INR 50 – 70 lakhs.
- Includes setting up dedicated power transformers, robust power backup systems (generators, UPS), main distribution panels, internal wiring, specialized lighting (e.g., non-shedding, cleanroom-compatible fixtures), and dedicated power lines for heavy machinery. Investing in energy-efficient systems can significantly reduce long-term operational costs.
- Fire Fighting & Safety Systems:
- Cost: INR 20 – 40 lakhs.
- Mandatory fire suppression systems (sprinklers, gas suppression for critical areas), fire alarms, smoke detectors, emergency lighting, and clearly marked emergency exits. Strict compliance with local fire safety regulations is non-negotiable and routinely audited.
- Environmental Control Systems:
- Cost: INR 30 – 60 lakhs.
- Effluent Treatment Plants (ETP): Essential for treating wastewater generated from manufacturing processes before discharge, adhering to stringent environmental norms. This can involve primary, secondary, and tertiary treatment stages.
- Air Pollution Control Devices: Scrubbers and dust collectors to manage emissions from certain processes, ensuring clean air standards.
- Hazardous Waste Management: Systems and protocols for safe collection, storage, and disposal of chemical and biological waste are critical for both compliance and environmental responsibility. Modern plants also increasingly focus on sustainability and green initiatives to reduce their environmental footprint.
2. Machinery and Equipment Costs
Investment Estimates: A Consolidated View
This represents a significant portion of the total investment, varying substantially based on the dosage form, desired production capacity, and the level of automation (semi-automatic vs. fully automatic).
- Production Machinery:
- Cost: INR 1 – 1.5 crores for a basic setup. Larger-scale operations or specialized products will incur significantly higher costs.
- Examples by Dosage Form:
- Oral Solid Dosage (Tablets/Capsules): High-shear granulators, fluid bed dryers, blenders (e.g., V-blenders, double cone blenders), tablet presses (rotary presses ranging from 10-50 lakhs depending on output), capsule filling machines (manual, semi-automatic, automatic, from 5-30 lakhs), tablet coating machines, and blister packing machines (10-40 lakhs).
- Liquid Orals/Syrups: Liquid filling machines, capping machines, labeling machines, and mixing tanks specific to liquid formulations.
- Injectables/Sterile Products: These are the most complex and expensive. They require aseptic filling lines (highly specialized and costly, starting from INR 1.5 crores), vial washing machines, sterilization tunnels, autoclaves, and lyophilizers (freeze dryers), which alone can cost INR 1-5 crores+ for high-capacity units.
- Quality Control (QC) Laboratory Equipment:
- Cost: INR 60 – 80 lakhs.
- Examples: This includes a wide array of precision instruments for raw material testing, in-process checks, and finished product analysis. Key equipment includes High-Performance Liquid Chromatography (HPLC) (INR 5-20 lakhs), Gas Chromatography (GC), dissolution testers, UV spectrophotometers, Karl Fischer titrators, stability chambers (for accelerated and long-term stability studies), and a fully equipped microbiology lab for sterility testing and microbial limit tests. Integrating these with a Laboratory Information Management System (LIMS) is crucial for data integrity.
- Utility Equipment:
- Cost: INR 30 – 50 lakhs.
- Includes industrial boilers (for steam generation), air compressors (for pneumatic operations), and chilling plants (for HVAC systems and process cooling).
- Material Handling Equipment:
- Cost: INR 10 – 20 lakhs.
- Pallet trucks, forklifts, scissor lifts, and conveyor systems for efficient movement of raw materials, in-process goods, and finished products within the facility.
3. Regulatory Approvals and Certifications
Navigating the complex regulatory landscape is crucial and incurs significant costs and time, but it fundamentally ensures product quality, safety, and market access.
- Licenses & Registrations:
- Manufacturing License: The primary license issued by the State Drug Control Department, permitting the legal manufacture of specific pharmaceutical products. The requirements can vary significantly for API manufacturing vs. formulation units, or sterile vs. non-sterile product lines.
- GMP (Good Manufacturing Practices) Certification: This is a fundamental quality system ensuring products are consistently produced and controlled according to quality standards. It covers all aspects of manufacturing, from starting materials, premises, and equipment to the training and personal hygiene of staff. Issued by the State Drug Control Department.
- WHO-GMP Certification: Essential for companies aiming to export products to many international markets or participate in government tenders. This certification signifies adherence to global standards, often requiring more stringent audits and demonstrating compliance with ICH guidelines.
- Other Essential Licenses: Drug sales license, consent from the Pollution Control Board, factory license, and a No Objection Certificate (NOC) from the Fire Safety Department.
- Approval Process Costs: These include application fees, inspection fees levied by regulatory bodies, and potentially significant consultant fees for dossier preparation, facility design review, and compliance guidance. A well-prepared dossier can significantly streamline the approval timeline.
- Estimated Cost: INR 10 – 15 lakhs.
- Timeline: The process can take 2-6 months for initial manufacturing and GMP licenses, with an additional 12 months or more required for WHO-GMP certification, depending on the preparedness and responsiveness of the applicant and regulatory bodies.
4. Minimum Initial Working Capital
This fund is absolutely essential to cover operational expenses during the crucial period before your plant achieves stable revenue generation and becomes self-sustaining. It acts as a critical financial buffer for the initial months of operation.
- Cost: INR 75 – 100 lakh.
- Components:
- Raw Materials & Packaging: Initial stock of Active Pharmaceutical Ingredients (APIs), excipients, solvents, empty bottles, vials, blister PVC, cartons, and labels. This inventory needs to be sufficient for a certain production cycle to avoid delays.
- Salaries & Wages: Covering the payroll for all staff for the initial 3-6 months of operation, before significant sales revenue starts flowing in.
- Utilities: Costs for electricity, water, fuel (for boilers/generators), and internet services.
- Consumables: Regular purchases for the QC lab (reagents, glassware), cleaning supplies, and general office supplies.
- Marketing & Distribution: Initial expenses for product launch, promotional activities, and establishing your distribution network.
5. Pre-Operating & Miscellaneous Expenses
These are often overlooked but vital costs incurred before commercial production begins. Neglecting these can lead to unexpected financial strain.
- Detailed Project Report (DPR) & Consultancy Fees: Engaging specialized consultants for feasibility studies, detailed engineering design, architectural planning, and regulatory affairs guidance is highly recommended. Their expertise can prevent costly errors and delays, providing significant long-term value.
- Legal & Professional Fees: Costs associated with company registration, drafting legal agreements, intellectual property registrations (if applicable), and other legal compliances.
- Software & IT Infrastructure: Investment in essential software systems like a Laboratory Information Management System (LIMS) for managing QC data, an Enterprise Resource Planning (ERP) system for overall business management (inventory, production, finance), and dedicated quality management software to ensure data integrity and compliance with regulatory requirements (e.g., 21 CFR Part 11). The integration of AI tools within these systems is increasingly vital for predictive analytics, process optimization, and ensuring compliance, marking a move towards Pharma 4.0.
- Contingency Fund:
- Cost: INR 10 – 20 lakhs.
- Importance: This is a crucial buffer for unforeseen delays, unexpected cost overruns, changes in regulatory requirements, or market fluctuations. A contingency fund of 10-15% of the total project cost is generally recommended to absorb these shocks without derailing the project. Don’t underestimate these “hidden” costs.
Here’s a consolidated estimate for setting up a pharmaceutical formulation plant in India, providing a realistic range across different scales. These figures are indicative and can vary significantly based on specific requirements, location, and the chosen product portfolio (e.g., injectables will be at the higher end of the range).
| Investment Category | Small Scale (INR) | Medium Scale (INR) | Sterile/Large Scale (INR) |
| Land & Infrastructure | 3 Cr – 5 Cr | 6 Cr – 10 Cr | 15 Cr – 25 Cr |
| Machinery & Equipment | 1.5 Cr – 3 Cr | 4 Cr – 7 Cr | 8 Cr – 20 Cr |
| Regulatory Approvals & Certifications | 10 L – 15 L | 15 L – 25 L | 25 L – 50 L |
| Minimum Initial Working Capital | 75 L – 1 Cr | 1.5 Cr – 2.5 Cr | 3 Cr – 5 Cr |
| Pre-Operating & Miscellaneous | 25 L – 50 L | 50 L – 1 Cr | 1 Cr – 2 Cr |
| Estimated Total Investment | 5.6 Cr – 9.65 Cr | 12.15 Cr – 21.25 Cr | 27.25 Cr – 52.5 Cr |
| Note: Costs are indicative and vary based on specific product lines, level of automation, and chosen technologies. |
Navigating Challenges: Risk Mitigation Strategies
Setting up a pharma plant is a complex undertaking, and anticipating potential roadblocks is crucial for success. Here are common challenges and strategies to mitigate them:
- Regulatory Delays: The regulatory landscape is dynamic. Diligent preparation of all documentation, adherence to every guideline, and engaging experienced regulatory consultants can significantly expedite approval processes.
- Cost Overruns: Unforeseen expenses are common. A detailed DPR with accurate financial projections and, critically, maintaining a robust contingency fund (10-15%) are your best defenses against unexpected costs.
- Market Volatility: Shifts in market demand or pricing can impact profitability. Develop a flexible production plan and a diverse product portfolio to adapt to market changes. Post-COVID, understanding supply chain resilience is more important than ever.
- Talent Acquisition & Retention: The demand for skilled pharmaceutical professionals is high. Invest in competitive compensation, continuous training, and fostering a positive work environment to attract and retain top talent.
- Compliance Changes: Regulatory requirements evolve. Establish a robust Quality Management System (QMS) that includes continuous monitoring of regulatory updates and a clear process for adapting operations accordingly.
- Supply Chain Disruptions: As seen during the pandemic, global supply chains can be vulnerable. Develop multiple supplier relationships, consider local sourcing where feasible, and implement predictive analytics for inventory management.
Frequently Asked Questions:
What is the typical timeframe for a pharma plant setup?
From initial planning to commercial launch, a small to medium-scale plant typically takes 1.5 to 2.5 years, with larger or sterile facilities requiring longer.
How important is location for cost?
Extremely. Land costs, labor availability, utility infrastructure, and local incentives vary significantly by region in India, directly impacting your initial investment and ongoing operational costs.
Can I scale up later?
Yes, future scalability should be a key consideration during the facility design & layout phase. Planning for modular expansion can save significant costs and disruption later.
What are the ongoing operational costs beyond initial setup?
Beyond working capital, anticipate continuous expenses for raw material procurement, salaries, utility bills, routine maintenance contracts, annual license renewals, continuous staff training, R&D investments, and ongoing quality assurance audits.
Is government support easily accessible?
Government schemes like PLI are well-structured. However, accessing them requires thorough documentation and adherence to specific criteria. Engaging consultants with expertise in navigating these schemes can be beneficial.
Conclusion
The opportunity in India’s pharmaceutical manufacturing sector is undeniable, offering a pathway to significant growth and global impact. While the investment required is substantial, a meticulously planned approach, guided by industry expertise, can transform your vision into a successful and compliant operation.
At Laafon, we bring deep industry knowledge, unparalleled regulatory expertise, and a forward-thinking approach that integrates modern technologies like AI and automation into your plant design and operations.
Ready to turn your vision into reality? Don’t navigate this complex journey alone. Contact Laafon Consultants today for a personalized feasibility study and expert guidance on your pharmaceutical manufacturing plant setup. Let’s build the future of pharma together.
Contact Us: email: contact@laafon.com Or Visit us: Contact
References:
- Business Today
- PIB.GOV.IN/Ministry of Commerce & Industry
- Phrma.org