Multiple myeloma, a complex and challenging hematologic malignancy, continues to pose significant therapeutic hurdles, particularly for patients with relapsed or refractory disease. Despite advancements in treatment, a substantial unmet need persists for patients who have exhausted conventional therapeutic avenues. The recent accelerated approval of LYNOZYFIC (linvoseltamab-gcpt) by the U.S. Food and Drug Administration (FDA) in July 2025 marks a pivotal moment in the treatment landscape for this patient population, offering a novel, targeted approach. This comprehensive article delves into the intricacies of LYNOZYFIC, providing healthcare professionals and informed pharma readers in India and the US with a detailed understanding of its mechanism, clinical efficacy, safety profile, and regulatory journey.
Also Read: Ibtrozi™ (Taletrectinib) | A New Era in ROS1-Positive NSCLC Treatment
Overview of the Drug: Introducing LYNOZYFIC (linvoseltamab-gcpt)
LYNOZYFIC, known generically as linvoseltamab-gcpt, represents a significant addition to the armamentarium against multiple myeloma. It is an innovative bispecific antibody designed to redirect the body’s own T-cells to target and eliminate myeloma cells. This therapeutic agent is administered as an intravenous injection, available in single-dose vials of 5 mg/2.5 mL (2 mg/mL) and 200 mg/10 mL (20 mg/mL). Its formulation is a clear to slightly opalescent, colorless to pale yellow solution, underscoring its sophisticated biotechnological origin.
The development of linvoseltamab-gcpt underscores a growing trend in oncology towards highly specific immunotherapies that leverage the patient’s immune system to combat cancer. Its unique mechanism of action positions it as a promising option for patients who have limited remaining treatment choices, offering a new ray of hope in a disease characterized by recurrent relapses and increasing refractoriness to successive lines of therapy.
Therapeutic Uses: Addressing the Unmet Need in Relapsed or Refractory Multiple Myeloma
Indication and Patient Population
LYNOZYFIC is specifically indicated for adult patients diagnosed with relapsed or refractory multiple myeloma. This designation implies that the disease has either returned after a period of remission (relapsed) or has not responded adequately to prior treatments (refractory). Crucially, the approval specifies a highly pre-treated patient population: those who have received at least four prior lines of therapy. This stringent criterion highlights the drug’s role as a late-line treatment option, reserved for patients with particularly challenging disease courses.
Furthermore, the prior therapy regimen must include three cornerstone classes of anti-myeloma agents: a proteasome inhibitor (PI), an immunomodulatory agent (IMiD), and an anti-CD38 monoclonal antibody. This requirement ensures that LYNOZYFIC is considered for patients whose disease has demonstrated resistance to established and widely used therapeutic modalities, thus addressing a critical unmet medical need.
Accelerated Approval and Clinical Benefit
The FDA’s approval of LYNOZYFIC falls under the accelerated approval pathway. This pathway is utilized for drugs that treat serious conditions and fill an unmet medical need, based on a surrogate endpoint that is reasonably likely to predict clinical benefit. In the case of LYNOZYFIC, the approval was granted based on compelling objective response rates (ORR) and the durability of these responses observed in clinical trials.
It is important for healthcare professionals to note that continued approval for this indication remains contingent upon the verification and description of clinical benefit in confirmatory trial(s). This regulatory nuance underscores the ongoing commitment to robust evidence generation and ensures that the long-term efficacy and safety profile of LYNOZYFIC are fully elucidated. For clinicians, this means staying abreast of ongoing research and confirmatory trial results, which will further solidify the drug’s place in treatment algorithms.
The target patient population for LYNOZYFIC is further defined by specific clinical characteristics. The efficacy population in the pivotal trial consisted of 80 patients who met the stringent prior therapy criteria. Patients with prior exposure to BCMA-directed bispecific antibody therapy, other bispecific T-cell engaging therapies, or BCMA CAR-T cell therapy were excluded, indicating that LYNOZYFIC is intended for those naive to this specific class of targeted immunotherapy. Patients enrolled in the study generally had a good performance status, with an Eastern Cooperative Oncology Group (ECOG) score of 0 or 1, and adequate baseline hematologic, renal, and hepatic function. The median age of 71 years (ranging from 37 to 83), with 30% of patients being 75 years or older, reflects the demographic reality of multiple myeloma, which predominantly affects older adults. This demographic insight is particularly relevant for clinicians in both India and the US, where an aging population necessitates therapies tailored to the unique considerations of geriatric patients.
Mechanism of Action: A Precision Strike Against Myeloma Cells
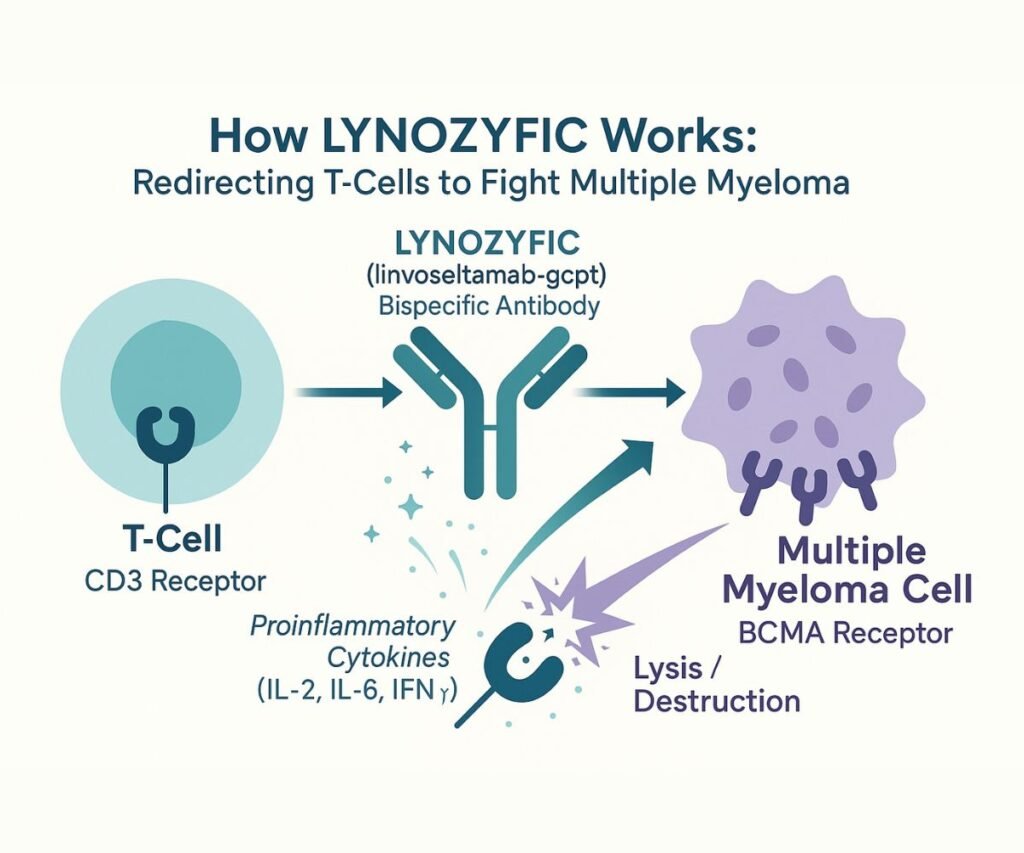
Linvoseltamab-gcpt’s therapeutic power lies in its sophisticated bispecific T-cell engager mechanism. This innovative class of antibodies represents a paradigm shift in cancer immunotherapy, moving beyond traditional monoclonal antibodies to directly harness the cytotoxic potential of T-cells.
Bispecific T-cell Engager Technology
At its core, linvoseltamab-gcpt is a recombinant human immunoglobulin (Ig) G4 antibody engineered to possess two distinct binding specificities. One arm of the antibody binds to the CD3 receptor, a crucial component of the T-cell receptor complex expressed on the surface of T-cells. The other arm specifically targets B-cell maturation antigen (BCMA), a protein highly expressed on the surface of multiple myeloma cells, as well as on some healthy B-lineage cells.
Targeted T-cell Activation and Myeloma Cell Lysis
The simultaneous binding of linvoseltamab-gcpt to both CD3 on T-cells and BCMA on myeloma cells creates an immunological synapse, effectively bringing the T-cell into close proximity with the tumor cell. This forced proximity is critical for initiating an anti-tumor response. Upon binding, linvoseltamab-gcpt activates the T-cells, leading to a cascade of events that includes:
- Release of Proinflammatory Cytokines: Activated T-cells release a variety of cytokines, such as interleukin-2 (IL-2), interleukin-6 (IL-6), and interferon-gamma (IFN-γ). These cytokines play a vital role in amplifying the immune response and creating a pro-inflammatory microenvironment that is detrimental to cancer cells.
- Lysis of Multiple Myeloma Cells: The activated T-cells directly engage and induce the lysis (destruction) of the BCMA-expressing multiple myeloma cells. This direct cytotoxic effect is the primary mechanism by which LYNOZYFIC exerts its anti-tumor activity.
Preclinical studies have further supported this mechanism, with linvoseltamab-gcpt demonstrating robust anti-tumor activity in mouse models of multiple myeloma. This dual-action mechanism—redirecting T-cells and inducing targeted cell death—highlights LYNOZYFIC’s precision and potential for deep and durable responses in a disease often characterized by diffuse involvement and resistance mechanisms. Understanding this intricate mechanism is paramount for healthcare professionals to anticipate and manage potential immune-related adverse events.
Dosage & Administration: A Carefully Orchestrated Regimen
The administration of LYNOZYFIC follows a meticulously designed dosage schedule aimed at optimizing efficacy while mitigating the risk of adverse events, particularly cytokine release syndrome (CRS) and neurologic toxicity. Adherence to these guidelines is critical for patient safety and treatment success.
Recommended Dosage Schedule
The treatment initiation involves a step-up dosing schedule, a common strategy with T-cell engagers to gradually introduce the drug and allow the immune system to adapt, thereby reducing the severity of initial immune activation.
- Step-up Doses:
- Day 1: 5 mg
- Day 8: 25 mg
- Day 15: 200 mg (This marks the first full treatment dose)
- Day 1: 5 mg
- Weekly Dosing: Following the step-up phase, patients receive 200 mg weekly for 10 doses, spanning from Week 4 to Week 13.
- Biweekly Dosing: From Week 14 onwards, the dosing frequency transitions to 200 mg biweekly (every 2 weeks).
- Reduced Frequency Dosing: For patients who achieve and maintain a very good partial response (VGPR) or better at or after Week 24 and have received at least 17 doses of 200 mg, the dosing frequency can be further decreased to 200 mg every 4 weeks. This individualized approach allows for reduced treatment burden for responders.
Important Administration Instructions and Premedication
Given the potential for severe immune-mediated reactions, specific administration protocols are mandatory:
- Hospitalization Requirement: Patients must be hospitalized for 24 hours after the administration of both the first step-up dose (5 mg) and the second step-up dose (25 mg). This critical measure ensures immediate access to emergency equipment and medical support for managing potential CRS and neurologic toxicity.
- Premedication: To minimize the risk of CRS and infusion-related reactions (IRR), patients are required to receive premedication prior to each dose of the step-up schedule (Day 1, Day 8, Day 15), the second treatment dose, and if indicated, subsequent treatment doses. The premedication regimen includes:
- Acetaminophen (or equivalent) 650 mg to 1,000 mg orally 30 to 60 minutes prior to infusion.
- Diphenhydramine (or equivalent) 25 mg orally or intravenously 30 to 60 minutes prior to infusion.
- Dexamethasone (or equivalent) intravenously 1 to 3 hours prior to infusion: 40 mg before step-up dose 1, step-up dose 2, and the first full treatment dose. Once a treatment dose is tolerated without CRS and/or IRR with 40 mg dexamethasone, 10 mg dexamethasone can be administered prior to subsequent doses. Pre-treatment medications may be discontinued once a treatment dose is tolerated without CRS and/or IRR.
- Preparation and Administration: LYNOZYFIC must be diluted in 0.9% Sodium Chloride Injection. It is crucial to use aseptic technique, and the vials are for single-use only. The solution should not be shaken. A 0.2-micron to 5-micron polyethersulfone (PES) filter is required for administration. LYNOZYFIC should not be mixed with other drugs or administered concurrently through the same intravenous line.
- Dose Modifications: Comprehensive guidelines are provided for managing adverse reactions, including dose delays, modifications, or permanent discontinuation, based on the severity of CRS, ICANS, or other adverse events. These detailed instructions are vital for safe and effective patient management in clinical practice.
Clinical Trials Summary: Evidence of Efficacy in LINKER-MM1
The efficacy of LYNOZYFIC was rigorously evaluated in the open-label, multi-center, multi-cohort study, LINKER-MM1 (NCT03761108). This pivotal trial provided the foundational data supporting the accelerated approval of linvoseltamab-gcpt.
Study Design and Patient Characteristics
The LINKER-MM1 study enrolled patients with relapsed or refractory multiple myeloma who had received at least three prior therapies, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 antibody. The efficacy population specifically focused on 80 patients who had received at least four prior lines of therapy, reflecting the heavily pre-treated nature of the target population. Patients had an ECOG performance status of 0 or 1 and adequate organ function.
Demographically, the median age of patients in the efficacy population was 71 years (range: 37 to 83), with a significant proportion (30%) being 75 years or older. The median number of prior lines of therapy was 5 (range: 4 to 13), and a striking 83% of patients were refractory to their last line of therapy. Furthermore, 79% of patients were triple-class refractory, highlighting the profound treatment resistance in this cohort. A subset of patients (13%) had also been previously treated with a BCMA antibody-drug conjugate. These patient characteristics underscore the challenging clinical scenario that LYNOZYFIC aims to address.
Key Efficacy Endpoints and Results
Efficacy was primarily established based on the objective response rate (ORR), as determined by a blinded independent review committee (IRC) using the International Myeloma Working Group (IMWG) criteria. The results demonstrated compelling anti-myeloma activity:
- Objective Response Rate (ORR): LYNOZYFIC achieved an ORR of 70% (95% CI: 59, 80). This high response rate in a heavily pre-treated population is clinically significant.
- Complete Response (CR) or Better: A substantial proportion of patients achieved deep responses, with 45% (95% CI: 34, 57) attaining a Complete Response (CR) or better. This included 39% achieving a Stringent Complete Response (SCR) and 6% achieving a CR.
- Very Good Partial Response (VGPR): An additional 19% of patients achieved a Very Good Partial Response.
- Partial Response (PR): 6% of patients achieved a Partial Response.
- Duration of Response (DOR): With a median follow-up of 11.3 months among responders, the estimated duration of response (DOR) was 89% at 9 months and 72% at 12 months. The median DOR was not estimable, suggesting sustained responses in a significant number of patients. The median time to first response was rapid, at 0.95 months (range: 0.5 to 6 months).
These robust efficacy data from LINKER-MM1 provide a strong rationale for LYNOZYFIC’s accelerated approval, offering a meaningful clinical benefit to patients with limited treatment options. The high rates of deep responses (CR or better) and the promising durability of response are particularly encouraging for managing this aggressive disease.
Adverse Events & Safety Profile: Navigating the Immunotherapy Landscape
Like many highly effective immunotherapies, LYNOZYFIC is associated with a distinct safety profile, dominated by immune-mediated adverse events. Comprehensive understanding and proactive management of these events are paramount for safe patient care.
Boxed Warning: Cytokine Release Syndrome (CRS) and Neurologic Toxicity
LYNOZYFIC carries a prominent Boxed Warning, highlighting two critical and potentially life-threatening adverse reactions:
- Cytokine Release Syndrome (CRS): This systemic inflammatory response can manifest with symptoms such as fever, chills, hypoxia, tachycardia, and hypotension. In LINKER-MM1, CRS occurred in 46% of patients, with most cases being Grade 1 (35%) or Grade 2 (10%). Grade 3 CRS occurred in less than 1% of patients. The median time to onset of CRS was 11 hours after the most recent dose, with a median duration of 15 hours. Management involves withholding LYNOZYFIC, providing supportive care (which may include intensive care), and modifying subsequent doses based on severity.
- Neurologic Toxicity, including Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS): Neurologic toxicity, including ICANS, occurred in 54% of patients, with Grade 3 or 4 events in 8%. ICANS specifically occurred in 8% of patients (Grade 3 in 2.6%). Common symptoms include confusion, depressed level of consciousness, and lethargy. The median time to onset of ICANS was 1 day after the most recent dose, with a median duration of 2 days. ICANS can occur concurrently with CRS, after CRS resolution, or in its absence. Management involves withholding LYNOZYFIC, comprehensive evaluation (including neurologist consultation), supportive therapy, and dose modification or permanent discontinuation based on severity. Patients are advised to refrain from driving or operating machinery for 48 hours after step-up doses or if neurologic symptoms occur.
Risk Evaluation and Mitigation Strategy (REMS)
Due to the significant risks of CRS and neurologic toxicity, LYNOZYFIC is available only through a restricted program known as the LYNOZYFIC REMS. This program ensures that the benefits of the drug outweigh its risks by implementing strict safety measures:
- Prescriber Certification: Healthcare providers prescribing LYNOZYFIC must be certified through the program by enrolling and completing specific training.
- Patient Counseling: Prescribers must counsel patients about the risks of CRS and neurologic toxicity and provide them with a LYNOZYFIC Patient Wallet Card, which outlines key symptoms and instructs patients to seek immediate medical attention if they experience them.
- Pharmacy and Healthcare Setting Certification: Pharmacies and healthcare settings dispensing LYNOZYFIC must also be certified and verify prescriber certification.
- Wholesaler and Distributor Restrictions: Wholesalers and distributors are restricted to distributing LYNOZYFIC only to certified pharmacies or healthcare settings.
Other Serious Warnings and Precautions
Beyond CRS and neurologic toxicity, other significant safety concerns include:
- Infections: LYNOZYFIC can cause serious, life-threatening, or fatal infections, including opportunistic infections. In LINKER-MM1, serious infections occurred in 42% of patients (Grade 3 or 4 in 38%), with fatal infections in 4%. Common serious infections included pneumonia and sepsis. Two cases of progressive multifocal leukoencephalopathy (PML) were reported. Monitoring for signs of infection and immunoglobulin levels, and administering prophylactic antimicrobials, are crucial.
- Neutropenia: Decreased neutrophil count occurred in 62% of patients (Grade 3 or 4 in 47%), and febrile neutropenia in 8%. Regular monitoring of complete blood cell counts is essential.
- Hepatotoxicity: Elevated liver enzymes (ALT in 46%, AST in 61%) and bilirubin (Grade 3 or 4 in 1.7%) can occur. Liver enzyme elevations can be independent of CRS. Monitoring liver enzymes and bilirubin is clinically indicated.
- Embryo-Fetal Toxicity: Based on its mechanism, LYNOZYFIC may cause fetal harm. Females of reproductive potential must use effective contraception during treatment and for 3 months after the last dose. Pregnancy status must be verified prior to initiation.
- Lactation: Women are advised not to breastfeed during treatment and for 3 months after the last dose due to the potential for serious adverse reactions in breastfed children.
- Drug Interactions: Linvoseltamab-gcpt can induce cytokine release, which may suppress CYP enzyme activity, potentially increasing exposure to certain CYP substrates. This risk is higher during the initial dosing phase and during CRS events.
Common Adverse Reactions and Laboratory Abnormalities
The most common adverse reactions (occurring in ≥20% of patients) included musculoskeletal pain, cytokine release syndrome, cough, upper respiratory tract infection, diarrhea, fatigue, pneumonia, nausea, headache, and dyspnea. The most common Grade 3 or 4 laboratory abnormalities (occurring in ≥30% of patients) were decreased lymphocyte count, decreased neutrophil count, decreased hemoglobin, and decreased white blood cell count. Serious adverse reactions occurred in 74% of patients, and fatal adverse reactions in 7%. Permanent discontinuation due to adverse reactions occurred in 16% of patients.
Pharmacodynamics & Pharmacokinetics: Understanding Drug Behavior
The pharmacodynamics (PD) and pharmacokinetics (PK) of linvoseltamab-gcpt provide critical insights into its activity and disposition within the body, guiding optimal dosing strategies.
Pharmacodynamics
The 200 mg once weekly dosing regimen was associated with superior objective response rates and complete response rates compared to a lower 50 mg once weekly regimen, underscoring the importance of adequate drug exposure for efficacy. A key pharmacodynamic effect observed was the transient elevation of circulating cytokines (IL-2, IL-6, and IFN-γ). This cytokine release primarily occurred during the step-up dose regimen and the first full 200 mg dose, with levels generally returning to baseline before the next dose. Limited cytokine release was observed with subsequent doses, indicating a potential desensitization or adaptation of the immune system over time. This cytokine profile is directly linked to the incidence of CRS and is a hallmark of T-cell engager therapies.
Pharmacokinetics
Linvoseltamab-gcpt’s PK profile was evaluated in patients with relapsed or refractory multiple myeloma at the recommended dosage.
- Exposure: The trough concentration (Ctrough) of linvoseltamab-gcpt increased more than proportionally over a dose range of 96 mg to 800 mg. The maximum concentration (Cmax) of 127 mg/L is achieved after the first dose of the every-2-weeks dosing regimen (i.e., the 12th dose of 200 mg).
- Distribution: The volume of distribution (Vd) for linvoseltamab-gcpt is 7.05 L, suggesting distribution primarily within the extracellular fluid compartment.
- Elimination: The clearance of linvoseltamab-gcpt is 0.68 L/day at baseline, decreasing to 0.43 L/day at steady state. This decrease in clearance over time is attributed to two parallel elimination processes: a linear, non-saturable catabolic process and a nonlinear, saturable target-mediated pathway. This target-mediated drug disposition (TMDD) implies that as the drug binds to its target (BCMA on myeloma cells), it is removed from circulation, leading to non-linear pharmacokinetics. Patients discontinuing linvoseltamab-gcpt are expected to have a 97% reduction from Cmax at a median time of 77.7 days (range: 18 to 154 days) after the last dose.
- Metabolism: Linvoseltamab-gcpt is expected to be metabolized into small peptides through catabolic pathways, typical for protein-based therapeutics.
- Specific Populations: No clinically significant differences in the pharmacokinetics of linvoseltamab-gcpt were observed based on age (37 to 91 years), weight, sex, race, ethnicity, mild to moderate renal impairment (creatinine clearance [CrCL] 30 to 89 mL/min), or mild hepatic impairment. However, the effect of severe renal impairment (CrCL 15 to 29 mL/min, end-stage renal disease) and moderate to severe hepatic impairment on the pharmacokinetics of linvoseltamab-gcpt remains unknown.
- Immunogenicity: During the LINKER-MM1 trial, anti-linvoseltamab-gcpt antibodies developed in 1% (2/192) of treated patients. Due to the low incidence, the clinical impact of these antibodies on pharmacokinetics, pharmacodynamics, safety, or effectiveness is not yet fully characterized.
Regulatory Path: A Journey Through Expedited Designations
The regulatory journey of LYNOZYFIC highlights its significance as a therapy for a high-need patient population, benefiting from several expedited review designations by the FDA.
Accelerated Approval and Expedited Designations
LYNOZYFIC received accelerated approval, a pathway reserved for drugs that address serious conditions and provide meaningful therapeutic advantages over existing treatments. This designation allowed for earlier patient access based on surrogate endpoints, with confirmatory trials ongoing. In addition to accelerated approval, LYNOZYFIC was granted several other expedited designations:
- Priority Review: This designation means the FDA’s goal is to take action on an application within 6 months, compared to 10 months for standard review, reflecting the urgency of getting this therapy to patients.
- Orphan Drug Designation: Granted for drugs intended to treat rare diseases or conditions affecting fewer than 200,000 people in the US. Multiple myeloma, while not extremely rare, qualifies for this designation due to its specific prevalence. This designation provides incentives for drug developers.
- Fast Track Designation: This designation is given to drugs that address unmet medical needs in serious conditions, facilitating the development and expedited review of new drugs.
Risk Evaluation and Mitigation Strategy (REMS)
As discussed, the LYNOZYFIC REMS program is a mandatory component of its approval, designed to manage the serious risks of CRS and neurologic toxicity. This structured program ensures that patients receive the drug under carefully controlled conditions, with appropriate monitoring and management strategies in place.
Post-Marketing Requirements and Advisory Committee Review
A crucial aspect of accelerated approval is the requirement for post-marketing clinical trials to verify and describe the clinical benefit. For LYNOZYFIC, the manufacturer is mandated to conduct a randomized trial comparing linvoseltamab-gcpt to standard therapy, with progression-free survival as the primary endpoint. This trial is scheduled for completion by December 2026, with the final report due by June 2027. This ongoing commitment to data generation ensures that the full clinical profile of LYNOZYFIC is established.
Notably, the application for LYNOZYFIC was not referred to an FDA advisory committee. This decision indicates that the FDA determined the application did not raise significant public health questions that would warrant a public discussion and recommendation from an external expert panel. This often occurs when the efficacy data are clear and the safety profile, while requiring management, is understood and mitigatable through a REMS program.
Manufacturer & Approval Milestones: Regeneron’s Contribution
LYNOZYFIC is manufactured by Regeneron Pharmaceuticals, Inc., a leading biotechnology company known for its innovative approaches to drug discovery and development. The approval of linvoseltamab-gcpt represents a significant milestone for Regeneron, expanding its oncology portfolio and reinforcing its commitment to addressing challenging diseases like multiple myeloma.
The drug substance is produced using recombinant DNA technology in Chinese hamster ovary (CHO) cell suspension culture, a standard and robust method for manufacturing complex biological therapeutics. The FDA’s approval of the stability protocols for extending the expiration dating period of both the drug substance and product is a testament to the rigorous quality control and manufacturing processes in place, ensuring the long-term availability and integrity of the medication.
The approval date of July 2, 2025, marks a critical juncture for patients with relapsed or refractory multiple myeloma, offering a new, targeted therapeutic option. This approval not only validates Regeneron’s scientific endeavors but also contributes to the evolving landscape of multiple myeloma treatment, which increasingly incorporates highly specific immunotherapies designed to overcome resistance and improve patient outcomes.
Summary
LYNOZYFIC (linvoseltamab-gcpt) represents a significant advancement in the treatment of relapsed or refractory multiple myeloma. Approved by the FDA in July 2025 under accelerated approval, it targets adult patients who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody. As a bispecific BCMA-directed CD3 T-cell engager, LYNOZYFIC redirects T-cells to specifically target and lyse myeloma cells, demonstrating a compelling objective response rate of 70% and a complete response or better rate of 45% in the pivotal LINKER-MM1 trial.
While highly effective, LYNOZYFIC’s safety profile necessitates careful management, particularly concerning the Boxed Warning for Cytokine Release Syndrome (CRS) and Neurologic Toxicity (including ICANS). These risks are managed through a mandatory REMS program, meticulous step-up dosing, and comprehensive premedication and monitoring protocols. Other significant adverse events include serious infections, neutropenia, and hepatotoxicity. The drug’s pharmacokinetics show a non-linear clearance influenced by target-mediated drug disposition, and its regulatory journey benefited from Priority Review, Orphan Drug, and Fast Track designations, underscoring the urgent unmet need it addresses. Manufactured by Regeneron Pharmaceuticals, Inc., LYNOZYFIC offers a new, targeted therapeutic avenue, with ongoing confirmatory trials poised to further define its long-term clinical benefit in this challenging patient population. Healthcare professionals in India and the US should familiarize themselves with its detailed administration, safety, and efficacy profile to optimize patient outcomes.
FAQs
What is the significance of the “gcpt” suffix in linvoseltamab-gcpt?
The “gcpt” suffix in linvoseltamab-gcpt is a unique four-letter suffix assigned by the United States Adopted Names (USAN) Council. These suffixes are used for non-proprietary names of monoclonal antibodies and related products to distinguish them from other similar biological products. They help in identifying the specific manufacturer or development program, ensuring pharmacovigilance and clear communication within the healthcare community, especially as more biosimilar and biobetter products enter the market.
References:
- Novel Drug Approvals for 2025-[US Food & Drug Administration]
- LYNOZYFIC Prescribing Information-[Regeneron]
- Phase 1/2 Study of REGN5458 in Adult Patients With Relapsed or Refractory Multiple Myeloma (LINKER-MM1)-[ClinicalTrials.gov]