The U.S. Food and Drug Administration (FDA) approved Grafapex treosulfan on January 22, 2025, marking a significant advancement in preparative regimens for allogeneic hematopoietic stem cell transplantation (alloHSCT) in patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) 310. Developed by Medac GmbH and commercialized in the U.S. by Medexus Pharmaceuticals, Grafapex offers a promising alternative to traditional conditioning therapies like busulfan, with improved survival rates and a manageable safety profile. This blog post explores the drug’s origin, mechanism, clinical applications, and key considerations for healthcare providers and patients.
Origin and Regulatory Background of Grafapex Treosulfan
- Developer and Approval: Grafapex was developed by the German pharmaceutical company Medac GmbH. Medexus Pharmaceuticals holds exclusive U.S. commercialization rights under a 2021 licensing agreement.
- FDA Approval: Approved on January 22, 2025, based on Phase III trial data (NCT00822393) demonstrating superior overall survival compared to busulfan.
- Orphan Drug Designation: Grants 7.5 years of market exclusivity in the U.S., enhancing accessibility for rare disease populations.
Also Read: Datroway (Datopotamab Deruxtecan) – A Breakthrough in Breast Cancer Treatment
Drug Category and Mechanism of Action of Grafapex treosulfan
Pharmacological Class
- Alkylating Agent: Grafapex (Treosulfan) belongs to the alkylating drug class, which damages DNA to inhibit cancer cell replication.
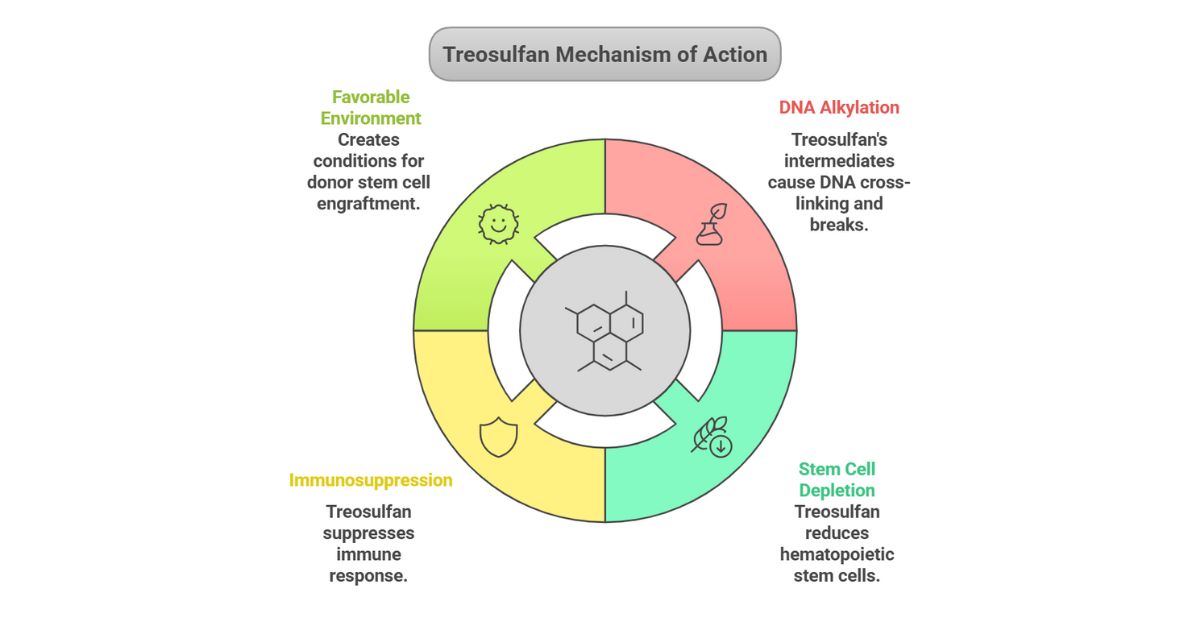
Mechanism of Action
- Prodrug Activation: Treosulfan is a water-soluble prodrug that converts into active epoxy intermediates (e.g., monoepoxides) under physiological pH (>5). These intermediates alkylate DNA, causing cross-linking and strand breaks, leading to apoptosis.
- Targeted Effects: Depletes hematopoietic stem cells and exerts immunosuppressive activity, creating a favorable environment for donor stem cell engraftment.
Clinical Uses and Indications of Grafapex (Treosulfan)
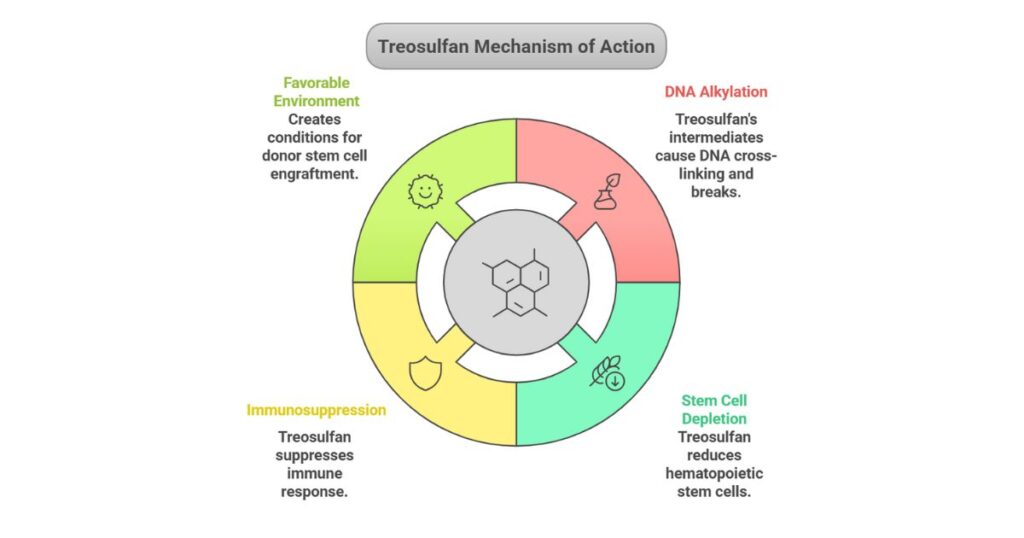
Grafapex (Treosulfan) is indicated for:
- Adult and Pediatric Patients (≥1 year) with:
- Acute Myeloid Leukemia (AML)
- Myelodysplastic Syndrome (MDS).
- Preparative Regimen: Used with fludarabine to condition patients before alloHSCT, replacing traditional therapies like busulfan.
Efficacy Highlights
- Trial MC-FludT.14/L (NCT00822393):
- 570 patients randomized to treosulfan (n=280) or busulfan (n=290).
- Hazard Ratio for Overall Survival: 0.67 (95% CI: 0.51–0.90), indicating a 33% reduction in mortality risk vs. busulfan.
- 2-Year Event-Free Survival: 64% (treosulfan) vs. 50.4% (busulfan).
Dosage and Administration of Grafapex treosulfan
Recommended Regimen
- Treosulfan: 10 g/m² IV daily on Days 4, 3, and 2 (relative to transplant Day 0).
- Fludarabine: 30 mg/m² IV daily on Days 6 to 2.
Preparation and Handling
- Reconstitution: Use 0.9% NaCl, 0.45% NaCl, or 5% dextrose. Avoid sterile water in children ≤12 years due to hypo-osmolarity risks.
- Infusion: Administer over 2 hours; monitor for extravasation.
Dosage Adjustments
- Renal/Hepatic Impairment: Limited data for moderate/severe cases; use caution
Safety Profile and Side Effects
Common Adverse Reactions (≥20%)
- Musculoskeletal pain (39%), stomatitis (38%), pyrexia (34%), nausea (33%), edema (29%).
Serious Risks
- Myelosuppression: Severe and prolonged cytopenias necessitate stem cell transplantation.
- Black Box Warning: Fatal complications are possible without HSCT; confirm donor availability before initiation.
- Secondary Malignancies: Linked to alkylating agents, particularly in patients with DNA repair disorders.
Precautions and Warnings
- Pregnancy and Lactation:
- Contraindicated due to genotoxicity; advise contraception for 6 months (females) and 3 months (males) post-treatment.
- Breastfeeding: Avoid during and for 1 week after therapy.
- Pediatric Considerations: Higher risk of hepatic/gastrointestinal adverse events; frequent diaper changes to prevent dermatitis.
- Drug Interactions: Weak inhibitor of CYP3A4 and CYP2C19; monitor substrates like anticoagulants or antidepressants.
Clinical Trial Insights
- Trial Design: Randomized, active-controlled study comparing treosulfan + fludarabine vs. busulfan + fludarabine.
- Key Outcomes:
- Improved OS in older/comorbid patients (HR 0.64–0.73).
- Lower incidence of severe infections (12% vs. 6% with busulfan).
Market Impact and Future Prospects
- Commercial Launch: The company is planning for H1 2025; projected to generate >$100 million annually within 5 years.
- Expanding Applications: Ongoing studies explore use in myelofibrosis and other leukemias.
Patient and Provider Resources for emergency situations
- Prescribing Information: Available on Drugs@FDA.
- Adverse Event Reporting: Use FDA MedWatch (1-800-FDA-1088).
- Contact Medac GmbH at 1-855-336 -3322 or FDA at 1-800-FDA-1088.
- www.fda.gov/medwatch.
If you want to buy the Grafapex you can contact below:
Ken d’Entremont | CEO, Medexus Pharmaceuticals
Tel: 905-676-0003 | Email: ken.dentremont@medexus.com
Conclusion
Grafapex Treosulfan represents a major advancement in conditioning therapy for hematopoietic stem cell transplantation (HSCT) in patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). Its enhanced safety profile, reduced hepatotoxicity, and effective myeloablative properties make it a strong alternative to conventional conditioning regimens.
With its recent FDA approval in 2025, Grafapex is expected to significantly impact transplant outcomes and improve survival rates, especially in patients who cannot tolerate traditional busulfan-based regimens.
FAQ
What is the expected cost of Grafapex, and will insurance cover it?
Grafapex’s exact pricing is not yet publicly disclosed, as the drug is scheduled for commercial availability by April 2025. However, its Orphan Drug Designation and 7.5-year market exclusivity in the U.S. may influence its cost, which is often higher for rare disease therapies. Medexus has indicated that annual revenue could exceed $100 million within five years, suggesting a premium pricing model.
Insurance Coverage: While specific formulary details are pending, Medexus is likely to work with insurers to ensure coverage, given its success with Trecondyv® in Canada, where public reimbursement agreements are already in place. Patients should consult their providers for plan-specific details or explore Medexus’s patient assistance programs post-launch.
How does Grafapex compare to busulfan in terms of cost-effectiveness and long-term outcomes?
Grafapex Treosulfan demonstrated superior efficacy in clinical trials, with a 33% reduction in mortality risk compared to busulfan and a 2-year event-free survival rate of 64% vs. 50.4%. While direct cost comparisons are unavailable, its improved survival rates and reduced severe infections (12% vs. 6%) may justify higher upfront costs through fewer complications and hospitalizations. Health economists will need to evaluate long-term cost-effectiveness as real-world data emerges
Are there specific insurance requirements for Grafapex eligibility (e.g., prior authorization)?
Though not explicitly stated, drugs like Grafapex Treosulfan often require prior authorization due to their specialized use in stem cell transplantation and high cost. Criteria may include confirmed AML/MDS diagnosis, donor availability, and failure of alternative therapies. Medexus plans to support healthcare providers with resources to navigate insurance processes, similar to its approach with Trecondyv® in Canada.
What pediatric-specific precautions are needed for Grafapex administration?
Pediatric patients (≥1 year) face higher risks of hepatic and gastrointestinal toxicities compared to adults. Recommendations include frequent diaper changes to prevent dermatitis (due to drug excretion in urine) and avoiding hypo-osmolar reconstitution fluids (e.g., sterile water) in children ≤12 years. Dosing remains weight-based at 10 g/m²/day, identical to adults.
How will Grafapex’s launch impact existing Medexus products like Trecondyv®?
Trecondyv® (Canada’s version of treosulfan) saw 55% unit demand growth in 2024, signaling strong market acceptance. Grafapex’s U.S. launch is expected to complement, not replace, Trecondyv®, as Medexus targets geographic expansion. The company’s infrastructure investments ($1.9 million in Q3 2025) aim to support both products without cannibalizing sales.
References
- GRAFAPEX (treosulfan) for injection-FDA(.gov)
- Medexus Pharmaceuticals. (2025). FDA Approval Announcement. (https://www.medexus.com/en_US/investors/news-events/press-releases/detail/176/medexus-announces-fda-approval-of-grafapex-treosulfan-for).
- RxList. (2025). Grafapex Drug Information.(https://www.rxlist.com/grafapex-drug.htm)
- Medscape. (2025). Treosulfan Dosing and Interactions.(https://reference.medscape.com/drug/grafapex-treosulfan-100168)
- ASCO. (2025). FDA Approval News Release(https://www.asco.org/news-initiatives/policy-news-analysis/fda-approves-treosulfan-fludarabine-preparative-regimen).
- Pharmacy Times. (2025). Clinical Trial Analysis. (https://www.pharmacytimes.com/view/fda-approves-treosulfan-regimen-for-preparative-use-in-stem-cell-transplantation-patients-with-aml-mds)