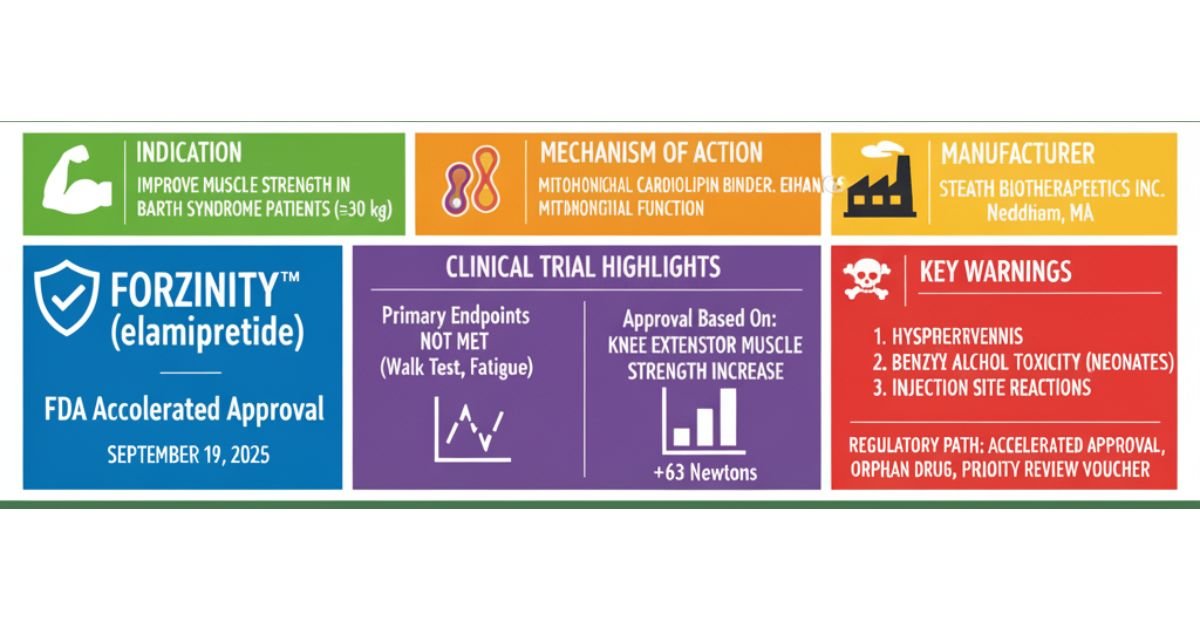
The U.S. Food and Drug Administration (FDA) granted Accelerated Approval to FORZINITY (elamipretide) on September 19, 2025, marking a pivotal moment as the first approved treatment for Barth Syndrome (BTHS) in eligible patients. This rare, X-linked, infantile-onset disease previously relied solely on supportive and symptomatic care.
FORZINITY functions as a first-in-class mitochondrial cardiolipin binder, aiming to restore compromised bioenergetics at the cellular level. The drug’s approval hinged on demonstrating an improvement in the intermediate clinical endpoint of knee extensor muscle strength, a measure assessed by Handheld Dynamometry (HHD).
Clinical data derived from the open-label extension (SPIBA-201, Part 2) confirmed sustained efficacy in this metric: the median increase in muscle strength was 34 newtons at Week 12 and reached 63 newtons by Week 168. This finding contrasts sharply with the initial randomized, placebo-controlled phase (SPIBA-201, Part 1), where the drug did not demonstrate superiority over placebo on the primary endpoints, such as the 6-minute walk test (p=0.97) and Total Fatigue Score (p=0.89).
Safety data showed the agent is generally tolerable, although the risk profile mandates vigilance. The most frequent adverse events were injection site reactions, including erythema and pain, occurring in up to 100% of treated patients. Clinicians must heed the boxed warning regarding the risk of Benzyl Alcohol Toxicity in Neonates as the formulation contains 20 mg/mL of benzyl alcohol. As required by the Accelerated Approval pathway, the sponsor must conduct a post-marketing randomized, double-blind trial to confirm the clinical benefit of the observed HHD improvement. This targeted therapeutic strategy offers new hope against BTHS pathogenesis.
The Molecular Rationale: Understanding Barth Syndrome Pathogenesis
Barth Syndrome (BTHS) is a severe, X-linked recessive genetic disorder characterized by defects in the TAFAZZIN gene. This molecular deficiency compromises the remodeling of cardiolipin, a critical phospholipid essential for maintaining the structural integrity and function of the inner mitochondrial membrane. The resulting mitochondrial dysfunction is linked to the complex pathogenesis seen in patients, manifesting primarily as cardiomyopathy, neutropenia, and skeletal muscle myopathy.
The Role of Mitochondrial Dysfunction in BTHS
- The underlying genetic error leads to an abnormal ratio of monolysocardiolipin to cardiolipin (MLCL:CL ratio), which is considered a biochemical hallmark of the disease.
- This cardiolipin abnormality compromises the efficient functioning of the electron transport chain , leading to impaired cellular bioenergetics and reduced production of adenosine triphosphate (ATP).
- Clinically, this energy deficit severely restricts muscle function, contributing to the poor stamina, exercise intolerance, and skeletal muscle weakness experienced by patients.
The Therapeutic Agent: FORZINITY (elamipretide)
Mechanism of Action: A Cardiolipin Binder
FORZINITY (elamipretide) introduces a novel therapeutic approach in the landscape of mitochondrial disease treatment.
- Target Specificity: Elamipretide is classified as a mitochondrial cardiolipin binder.
- Cellular Localization: The agent localizes to the inner mitochondrial membrane , the site of compromised cardiolipin metabolism in BTHS.
- Functional Outcome: By binding to cardiolipin, the drug acts to improve mitochondrial morphology and function, a strategy aiming to rescue ATP synthesis and restore cellular bioenergetics. This is a crucial area of biotech innovation in mitochondrial medicine.
Pharmacokinetics and Metabolism
The disposition of elamipretide demonstrates properties consistent with a peptide-based drug administered via injection.
- Absorption and Distribution: Following subcutaneous administration, elamipretide exhibits rapid uptake, reaching maximum plasma concentrations (Cmax) between 0.5 to 1 hour. Absolute bioavailability is approximately 92%. The drug distributes throughout total body water, with a volume of distribution of about 0.5 L/kg.
- Metabolism: Metabolism occurs via sequential C-terminal degradation , yielding the inactive M1 tripeptide and M2 dipeptide metabolites. Notably, hepatic metabolism was not observed in vitro , indicating a non-CYP clearance pathway.
- Elimination: Elamipretide and its metabolites are primarily excreted in the urine , with approximately 100% of the dose recovered in the urine within 48 hours in patients with normal renal function. Minimal accumulation of the parent drug occurs with daily dosing.
Renal Impairment and Dosing Considerations
The clearance pathway necessitates dose adjustments in patients with impaired renal function.
- Exposure Increase: Exposure (AUC) to elamipretide increases proportionally with the severity of renal impairment, increasing by 125% in subjects with severe renal impairment (creatinine clearance <30 mL/min) not on dialysis.
- Metabolite Accumulation: The exposure of the inactive metabolites M1 and M2 sees marked increases in the severe renal impairment group (up to 280% and 640%, respectively).
- Dosage Guidance: Based on these PK findings, the required dosage is reduced to 20 mg subcutaneously once daily for adults with severe renal impairment (eGFR <30 mL/minute) and not on dialysis.
Clinical Data and Efficacy Metrics
The clinical development program assessed the impact of daily subcutaneous elamipretide (40 mg) in male patients aged 12 to 35 years with Barth syndrome. The program included a randomized, placebo-controlled crossover study (Part 1) followed by a long-term open-label extension (Part 2).
SPIBA-201 Part 1:
The primary efficacy objectives focused on functional and symptomatic improvement over a 12-week treatment period:
- Primary Endpoint Failures: FORZINITY was not superior to placebo on either of the co-primary endpoints:
- Distance walked during 6MWT: p=0.97.
- Total Fatigue Score (Barth syndrome Symptom Assessment): p=0.89.
SPIBA-201 Part 2:
The approval was predicated on the robust, long-term findings from the uncontrolled extension phase regarding skeletal muscle function.
- Efficacy Endpoint (Intermediate): Knee extensor muscle strength measured by HHD demonstrated an important signal in the extension phase.
- Magnitude and Durability of Effect: Patients experienced clinically meaningful gains that were sustained over more than three years:
- Median Change at Week 12 (open-label): 34 newtons.
- Median Change at Week 72: 35 newtons.
- Median Change at Week 168: 63 newtons.
The Therapeutic Landscape: Positioning the New Agent
The introduction of FORZINITY marks a transition from purely symptomatic management to a targeted therapeutic strategy within Barth syndrome treatment. As FORZINITY is the first approved drug for this rare disease, the comparison defaults to the historical Standard of Care (SoC).
FORZINITY vs. Standard of Care (SoC) for BTHS
| Feature | FORZINITY (elamipretide) | Standard of Care (SoC) [Historically] | ||
| Therapeutic Class | Mitochondrial cardiolipin binder (Novel) | Supportive/Symptomatic management | ||
| Primary Goal | Improve muscle strength by correcting mitochondrial | dysfunction | Treat manifestations (e.g., heart failure, neutropenia) | |
| Efficacy Data | Objective gain in | HHD muscle strength in uncontrolled long-term follow-up | Focus on managing clinical | morbidity (e.g., HF meds, G-CSF for neutropenia) |
| Innovation | First drug approved for Barth Syndrome (New | Molecular Entity) | Conventional, established therapies |
Clinical Integration and Treatment Algorithms
The approval signals a shift toward addressing the underlying cellular bioenergetics of the disease.
- Adjunctive Therapy: FORZINITY will likely be integrated as an adjunctive therapy to address muscle weakness, a central Barth syndrome manifestation. Current management of cardiac and hematologic issues (e.g., neutropenia, which increases infection risk) remains reliant on conventional SoC (e.g., G-CSF, heart failure drugs).
- Accelerated Pathway Risk: The accelerated approval status is acknowledged, highlighting the need for HCPs and patients to understand that the approval is based on a surrogate endpoint (HHD muscle strength) reasonably likely to predict benefit, not confirmed long-term clinical outcomes (e.g., survival or quality of life).
- Focus of Barth Syndrome Research: The drug’s success, even through an accelerated pathway, strongly validates the focus on mitochondrial targeted therapeutics for rare mitochondrial myopathy conditions.
Safety Profile, Warnings, and Contraindications
While addressing an urgent unmet need, adherence to the safety protocol is paramount, particularly given the drug’s composition and the vulnerable nature of the target population.
Benzyl Alcohol Toxicity
A critical warning involves the presence of benzyl alcohol as a preservative in the injectable solution:
- Neonatal Risk (Quote): The drug is contraindicated in neonates, with the explicit warning: “FORZINITY is not approved for use in neonates.”.
- Gasping Syndrome: The risk stems from the potential for serious, even fatal, adverse reactions, including gasping syndrome, a neurotoxicity associated with IV administration of benzyl alcohol in preterm or low-birth weight neonates.
- Concentration: FORZINITY contains 20 mg of benzyl alcohol per mL.
Hypersensitivity and Local Reactions
Hypersensitivity represents another significant safety concern that warrants careful monitoring:
- Contraindication (Quote): The drug is contraindicated in any patient with “Serious hypersensitivity to elamipretide or any of the excipients in FORZINITY [see Warnings and Precautions (5.2)].”.
- Reaction Manifestations: Documented hypersensitivity reactions include rash, papular lesions, and eczematous dermatitis, potentially occurring within minutes to months after initiating treatment.
- Administration Site: The most common adverse reactions across all patients are injection site reactions. These include erythema, pain (75%), and induration/pruritus (67%). HCPs must instruct patients to rotate the injection site daily and avoid injecting into tender, bruised, or scarred skin.
Eosinophilia and Renal Impact
- Hematologic Finding: Increases in absolute eosinophil counts were a frequent finding in the trials, typically peaking around 90 days after initial exposure. Importantly, this laboratory elevation was not associated with clinical manifestations or changes in other laboratory parameters.
- Renal Function Monitoring: Dose adjustment is required for adults with severe renal impairment due to impaired clearance and accumulation of the drug and its metabolites.
Dosage and Administration: Practical Guidance for HCPs
The NDA included detailed guidance on safe and effective dosing, emphasizing the fixed dose regimen and critical administration techniques.
Dosing and Population
- Fixed Dose: The standard dose is 40 mg subcutaneously once daily.
- Weight Threshold: This dose is approved only for patients weighing at least 30 kg.
- Preparation and Use: FORZINITY is supplied as a ready-to-use clear, colorless to yellow sterile aqueous solution. Clinicians must instruct patients/caregivers to inspect the vial and discard if the solution is cloudy or contains particulate matter.
Storage and Handling
- Storage: Unopened vials must be stored refrigerated at 2∘C to 8∘C (36∘F to 46∘F). Do not freeze.
- In-Use Stability: After the first dose, the opened vial may be stored under refrigeration (2∘C to 8∘C) or at room temperature (20∘C to 25∘C).
- Discard Policy: HCPs must ensure patients are aware of the strict discard instruction: Discard vials 8 days after first opening.
Global Access for FORZINITY (elamipretide) in India
As FORZINITY recently received Accelerated Approval by the U.S. FDA, its availability within India is determined by the local regulatory process (DCGI) and subsequent distribution plans. The unique classification as a rare disease therapy highlights a significant need for expedited access across the globe. Patients and healthcare providers in India treating Barth Syndrome can consult our website for the latest updates on regulatory filings and patient access programs for this mitochondrial therapeutic agent. For the most current information regarding importation, compassionate use, and anticipated launch timelines in the Indian market, visit:
Conclusion
The Accelerated Approval of FORZINITY for Barth Syndrome represents a significant forward step in the treatment of rare mitochondrial myopathy. While the initial randomized trial phase did not achieve primary endpoint goals, the compelling, sustained gain in skeletal muscle function observed over the long-term extension provided the evidentiary threshold for the FDA’s decision under the Accelerated Approval mechanism. This distinction is crucial for HCPs and investors alike in evaluating the drug’s therapeutic and commercial risk profiles.
The approval validates targeted mitochondrial therapeutics as a viable drug development strategy. The future of FORZINITY hinges on the successful execution of the mandated post-marketing confirmatory trial (due March 2030) to definitively verify the translation of improved HHD muscle strength into meaningful clinical benefit for Barth syndrome patients. Meanwhile, the drug’s first-in-class status and rare pediatric designation position it as a key entry point for treating the Barth syndrome pathogenesis at its mitochondrial root.
FAQ
Why was FORZINITY™ granted Accelerated Approval if it didn’t meet its primary endpoints?
The FDA granted accelerated approval based on the drug’s ability to improve knee extensor muscle strength, which was considered a meaningful intermediate clinical endpoint. While the primary endpoints for walking distance and fatigue were not met, the improvement in muscle strength was deemed sufficient for this approval pathway, pending a confirmatory trial.
Is every patient with Barth syndrome eligible for FORZINITY™?
No, the indication is specifically for adult and pediatric patients with Barth syndrome who weigh at least 30 kg. The drug’s safety and effectiveness have not been established in children weighing less than 30 kg , and it is not approved for use in neonates due to the risk of benzyl alcohol toxicity.
What is the most common side effect patients should expect?
Injection site reactions are the most common side effects. In the main clinical trial, 100% of patients treated with FORZINITY™ experienced injection site redness (erythema), and 75% experienced injection site pain.
Are there any dosage adjustments for specific patient groups?
Yes, the dosage should be reduced by half, from 40 mg to 20 mg once daily, for adult patients with severe renal impairment (eGFR less than 30 mL/minute) who are not on dialysis.
What should a patient do if they miss a dose?
The patient should skip the missed dose and take the next dose at the regularly scheduled time. A double dose should not be taken to make up for the missed one.
References:
- Elamipretide Clinical Trials. Stealth BioTherapeutics, Inc. Accessed October 4, 2025. www.stealthbt.com/programs-pipeline/
- FDA Grants Accelerated Approval to First Treatment for Barth Syndrome. U.S. Food and Drug Administration (FDA) News Release. September 19, 2025.
- Forzinity (elamipretide) injection label. U.S. Food and Drug Administration (FDA) AccessData. Revised September 2025. NDA 215244.
- Karaa, A., et al. Efficacy and Safety of Elamipretide in Individuals With Primary Mitochondrial Myopathy: The MMPOWER-3 Randomized Clinical Trial. Neurology, 2023, 101(3): e238-e252.
- Joffe, H.V. NDA 215244 Integrated Review. NDA 215244 Submission Documents. U.S. Food and Drug Administration (FDA). May 15, 2025.
- Vernon, H.,et al. FDA approves drug for treatment of rare mitochondrial disorder. JHU Hub. September 25, 2025. Accessed October 4, 2025$.
- Elamipretide: A Review of Its Structure, Mechanism of Action, and Therapeutic Potential. Int J Mol Sci. 2025 Feb 1.
- Stealth BioTherapeutics Announces FDA Accelerated Approval of Forzinity (elamipretide HCl), the First Therapy for Progressive and Life-limiting Ultra-rare Genetic Disease Barth Syndrome. PR Newswire. September 19, 2025.
- Lambert, C. M. NDA 215244 Risk Assessment and Risk Mitigation Review. U.S. Food and Drug Administration (FDA). NDA 215244 Submission Documents. January 28, 2025.
- FDA Grants Rare Pediatric Disease Designation to Stealth BioTherapeutics for Elamipretide for the Treatment of Barth Syndrome. PR Newswire. March 3, 2020.