MYQORZO (aficamten) represents a significant advancement in the management of obstructive hypertrophic cardiomyopathy (oHCM), a condition characterized by thickened heart muscle that obstructs blood flow out of the heart. Approved by the FDA in December 2025, it provides a targeted therapeutic option for patients burdened by the debilitating symptoms of this disease. In the current HCM management landscape, MYQORZO matters because it offers a precise mechanism to reduce cardiac contractility and relieve left ventricular outflow tract (LVOT) obstruction.
Drug Overview at a Glance
- Brand Name: MYQORZO™
- Generic Name: aficamten
- Drug Class: Cardiac myosin inhibitor
- Route of Administration: Oral
- FDA Approval Date: December 2025
- Approved Indication: Treatment of adults with symptomatic oHCM to improve functional capacity and symptoms
- Manufacturer: Cytokinetics, Inc.
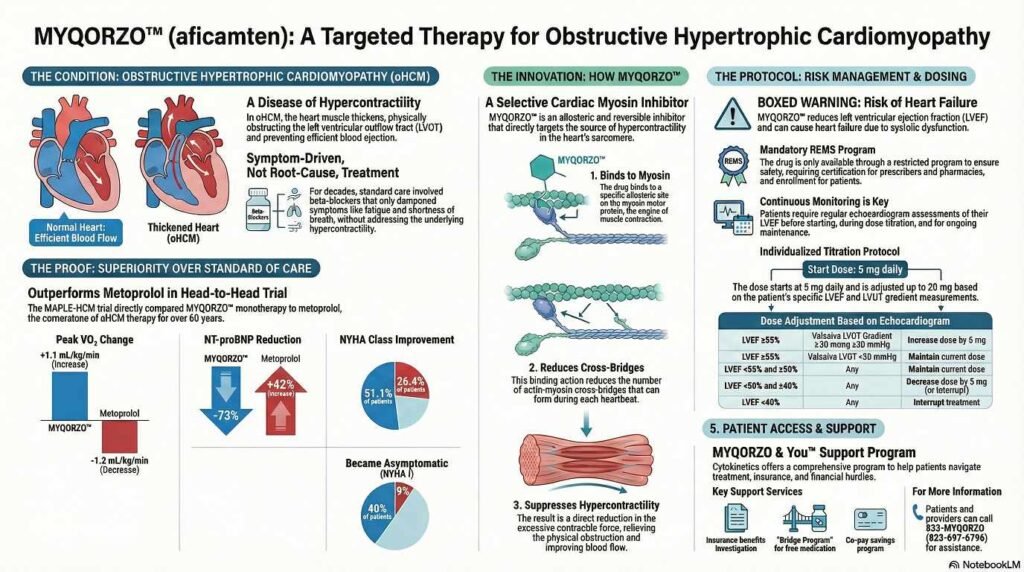
What Is MYQORZO?
MYQORZO is a first-in-class allosteric and reversible inhibitor of cardiac myosin motor activity. It is specifically indicated for adults with symptomatic obstructive hypertrophic cardiomyopathy (oHCM). By directly targeting the underlying pathophysiology of HCM—excessive myosin-actin cross-bridging—it positions itself as a specialized medical therapy alongside traditional options like beta-blockers or calcium channel blockers.
Mechanism of Action (MOA)
Myqorzo (aficamten) acts as a cardiac myosin inhibitor designed to treat obstructive hypertrophic cardiomyopathy (oHCM) by directly targeting the heart muscle’s excessive squeezing capability.
Its mechanism of action involves the following key processes:
• Reducing Molecular Connections: In patients with oHCM, genetic mutations cause the heart muscle to form too many connections—known as actin-myosin cross-bridges—during each heartbeat,. Myqorzo binds to a specific site on the cardiac myosin protein, preventing it from entering a force-producing state,. This action reduces the number of active cross-bridges involved in each contraction.
• Suppressing Hypercontractility: By reducing these cross-bridges, Myqorzo suppresses the pathological hypercontractility (excessive contraction) characteristic of oHCM.
• Relieving Obstruction: This reduction in contractile force decreases the obstruction in the left ventricular outflow tract (LVOT), allowing the heart to pump blood more efficiently to the rest of the body and improving functional capacity.
Myqorzo is described as a selective, allosteric, and reversible inhibitor,. It was engineered to have a rapid onset of action and a predictable exposure-response profile, with effects that diminish quickly (typically within two weeks) after the medication is stopped
FDA-Approved Indications
- Indication: Treatment of adults with symptomatic obstructive hypertrophic cardiomyopathy (oHCM) to improve functional capacity and symptoms.
- NYHA Class Eligibility: Symptomatic New York Heart Association (NYHA) class II and III.
- Inclusion/Exclusion: Initiation in patients with a left ventricular ejection fraction (LVEF) <55% is not recommended. Patients with certain infiltrative or storage disorders (e.g., Fabry disease, amyloidosis) were excluded from pivotal trials.
Dosage Forms and Strengths
MYQORZO is available as film-coated tablets in four strengths:
- 5 mg: Purple, round tablet.
- 10 mg: Purple, triangular tablet.
- 15 mg: Purple, pentagonal tablet.
- 20 mg: Purple, oval tablet.
Recommended Dosage and Administration
- Starting Dose: 5 mg orally once daily.
- Titration Strategy: Increase the dose every 2 to 8 weeks by 5 mg increments based on echocardiographic assessments of LVEF and Valsalva LVOT-G.
- Maximum Dose: 20 mg once daily.
- Dose Adjustment: Maintain LVEF ≥50%; if LVEF falls between 40% and 50%, the dose must be decreased.
- Interruption: Interrupt treatment for at least 7 days if LVEF <40% or if the patient experiences worsening clinical status.
Pharmacokinetics (PK)
1 Absorption
- Tmax: Median time to maximum concentration is 1.5 to 2.0 hours.
- Food Effect: No clinically significant differences in exposure when taken with a high-fat, high-calorie meal.
2 Distribution
- Protein Binding: Approximately 90% bound to plasma proteins.
- Volume of Distribution: 313 L.
3 Metabolism
- Primary Pathways: Extensively metabolized primarily through CYP2C9, with contributions from CYP3A, CYP2D6, and CYP2C19.
- Metabolites: Two major inactive metabolites, CK-3834282 and CK-3834283.
4 Elimination
- Half-life: Approximately 80 hours in oHCM patients.
- Excretion: 32% in urine and 58% in feces.
5 Special Populations
- Renal/Hepatic: No clinically meaningful PK differences in mild-to-moderate impairment. Effects in severe impairment are unknown.
- Demographics: Exposure is ~31% higher in females and ~34% lower in the highest body weight quartile, though these are not considered clinically significant.
Pharmacodynamics (PD)
MYQORZO causes dose-proportionate reductions in the LVOT gradient. It also impacts cardiac biomarkers:
- NT-proBNP: 80% lower than placebo at Week 24.
- Troponin I: 40% lower than placebo at Week 12 and 24.
- LVEF: Reductions are consistent with the mechanism of action, returning to baseline after treatment discontinuation.
Clinical Trial Program Overview
The MYQORZO clinical development program included the pivotal SEQUOIA-HCM Phase 3 trial and the ongoing FOREST-HCM long-term safety study. These trials established its efficacy in improving exercise capacity and symptoms in patients with oHCM.
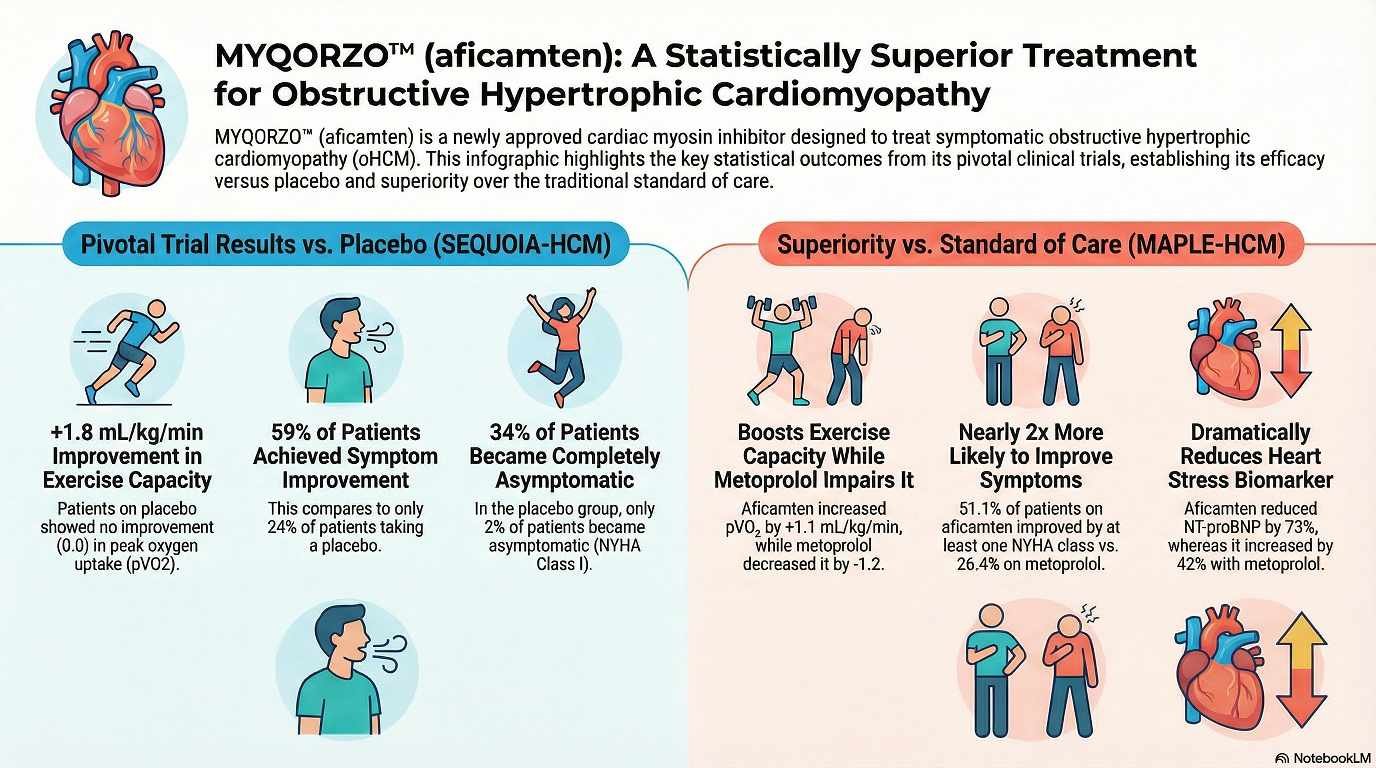
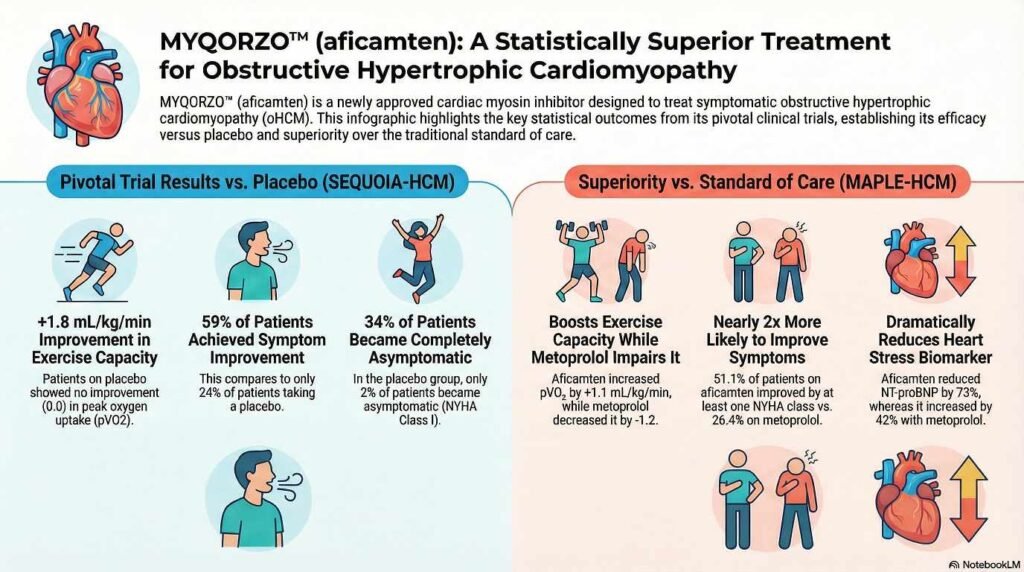
Pivotal Phase 3 Trial: SEQUOIA-HCM
1 Study Design
A multicenter, randomized, double-blind, placebo-controlled Phase 3 study involving 282 adults treated for 24 weeks.
2 Patient Population
- Inclusion: Adults with symptomatic NYHA class II/III oHCM and LVEF ≥60%.
- Background Therapy: 61% were on beta-blockers and 29% on calcium channel blockers.
3 Primary Endpoint
Change from baseline in peak oxygen uptake () by cardiopulmonary exercise testing (CPET) at Week 24.
4 Key Efficacy Results
- Primary Outcome: MYQORZO significantly improved by 1.7 mL/kg/min compared to placebo ().
- Secondary Outcomes: Significant improvements were seen in NYHA class (58.5% vs. 24.3%), KCCQ-CSS scores, and reductions in Valsalva LVOT-G.
5 Safety Outcomes
Overall, MYQORZO was well-tolerated. Hypertension (8% vs. 2%) was the most common adverse reaction. Reversible LVEF reduction to <50% occurred in 4% of patients but was not associated with clinical heart failure.
Comparative Clinical Trial: MAPLE-HCM
MAPLE-HCM was a pivotal Phase 3, randomized, double-blind, active-comparator clinical trial designed to evaluate the safety and efficacy of aficamten (Myqorzo) monotherapy compared directly to metoprolol (a standard-of-care beta-blocker) in adults with symptomatic obstructive hypertrophic cardiomyopathy (oHCM).
Key Study Details:
• Population: 175 adults with symptomatic oHCM were randomized to receive either aficamten (5–20 mg daily) or metoprolol (50–200 mg daily).[4]
• Primary Endpoint: The trial measured the change in peak oxygen uptake (pVO2) at 24 weeks[4].
Key Results:
• Exercise Capacity: Aficamten was superior to metoprolol. Patients taking aficamten achieved an increase in pVO2 of 1.1 mL/kg/min, while those on metoprolol experienced a decrease of 1.2 mL/kg/min (statistically significant difference of 2.3 mL/kg/min)[4].
• Symptom Improvement: Significantly more patients on aficamten saw an improvement of at least one New York Heart Association (NYHA) functional class compared to metoprolol (51% vs. 26%)[4].
• Hemodynamics: Aficamten resulted in profound reductions in LVOT gradients (obstruction) and NT-proBNP (a cardiac stress biomarker), whereas metoprolol did not effectively lower LVOT gradients despite reducing heart rate.
• Safety: The adverse event profile was similar between the two groups, with serious adverse events occurring in 8.0% of the aficamten group versus 6.9% of the metoprolol group.
Clinical Significance: This trial provided the first head-to-head evidence that a cardiac myosin inhibitor could outperform the standard-of-care beta-blocker. The results suggest that aficamten targets the underlying pathophysiology of oHCM more effectively than beta-blockers, potentially reshaping first-line treatment guidelines for the disease.
Ongoing and Future Clinical Studies
Patients from SEQUOIA-HCM are eligible for FOREST-HCM, an ongoing, open-label, long-term safety study.
Safety Profile
1 Boxed Warning: Risk of Heart Failure
MYQORZO reduces LVEF and can cause heart failure due to systolic dysfunction. Echocardiograms are required before and during treatment.
2 Contraindications
Concomitant use of rifampin is contraindicated.
3 Warnings and Precautions
- Heart Failure Risk: Increased risk with intercurrent illness (e.g., serious infection) or new arrhythmias.
- LVEF Monitoring: Required for safe titration and maintenance.
REMS Program Requirements
Due to the heart failure risk, MYQORZO is only available through the MYQORZO REMS Program.
- Prescribers: Must be certified by reviewing education and passing a knowledge assessment.
- Patients: Must enroll and comply with regular echocardiogram monitoring.
- Pharmacies: Must be certified and only dispense to authorized patients.
Drug–Drug Interactions
- Inhibitors: Strong CYP2C9 inhibitors or drugs inhibiting multiple pathways (e.g., fluconazole, voriconazole, fluvoxamine) can increase aficamten levels and heart failure risk.
- Inducers: Moderate-to-strong CYP3A inducers can decrease effectiveness.
Use in Special Populations
- Pregnancy: No human data; animal studies showed potential for structural malformations at high exposures.
- Lactation: It is unknown if aficamten passes into human milk.
- Pediatric: Safety and effectiveness not established.
- Geriatric: No overall differences in safety or effectiveness in patients ≥65 years.
MYQORZO Dosing and Titration Guide
The maintenance dose of MYQORZO is individualized for each patient based on echocardiographic assessments of the Left Ventricular Ejection Fraction (LVEF) and Valsalva Left Ventricular Outflow Tract Gradient (LVOT-G).
Table 1: Dose Adjustment Criteria
| LVEF Status | Valsalva LVOT-G | Action Required |
| Greater than 55% | Greater than 30 mmHg | Increase dose by 5 mg (Max: 20 mg once daily) |
| Greater than 55% | <30 mmHg | Maintain current dose |
| <55% to Greater than 50% | Any | Maintain current dose |
| <50% to Greater than 40% | Any | Decrease dose by 5 mg |
| <40 % | Any | Interrupt treatment for at least 7 days |
*Note: If a patient is already on the minimum 5 mg dose and LVEF falls between 40%-50%, treatment must be interrupted for at least 7 days.
Key Clinical Protocols for Titration
- Starting Dose: All patients begin at 5 mg orally once daily.
- Titration Frequency: Dose increases may occur every 2 to 8 weeks in 5 mg increments until the target maintenance dose or the maximum 20 mg dose is reached.
- Echocardiogram Timing: Assessments must be performed 2 to 8 weeks after initiation, after any dose adjustment, or after a treatment interruption.
- Resuming After Interruption: If treatment was paused due to low LVEF (<55%), it may be resumed at the 5 mg starting dose only once the LVEF recovers to greater than 55%.
- Maintenance Monitoring:
- For patients with LVEF greater than 55%: Monitor every 6 months.
- For patients with LVEF qual or <55% to greater than 50%: Monitor every 3 months.
- Administration: Tablets should be swallowed whole once daily, with or without food, at approximately the same time each day.
Warning for Intercurrent Illness
In patients experiencing a serious intercurrent illness (such as a severe infection or COVID-19) or a new arrhythmia, do not increase the dose until the condition has stabilized or resolved.
Comparison With Existing Therapies
MYQORZO provides a targeted mechanism compared to traditional agents like beta-blockers or disopyramide, which do not address the primary motor protein dysfunction. It significantly reduced the duration of eligibility for septal reduction therapy (SRT) by 78 days compared to placebo.
Pricing, Access, and Availability
MYQORZO is distributed by Cytokinetics, Inc.. Availability is restricted to certified specialty pharmacies under the REMS program.
Frequently Asked Questions (FAQs)
Who should receive MYQORZO?
Adults with symptomatic NYHA Class II-III oHCM.
How often is monitoring required?
Every 2-8 weeks during titration; then every 3-6 months depending on LVEF status.
Can it be taken with food?
Yes, with or without meals.
Key Takeaways for Clinicians
MYQORZO is a potent, reversible cardiac myosin inhibitor that significantly improves exercise capacity and NYHA class in oHCM patients. Clinicians must adhere to the REMS protocol, ensuring regular echocardiographic monitoring to manage the inherent risk of systolic dysfunction.
Conclusion
The approval of MYQORZO marks a milestone in oHCM therapy, offering a non-invasive means to achieve significant hemodynamic and symptomatic relief. Its integration into clinical practice requires a rigorous monitoring framework to balance its profound efficacy with cardiovascular safety.
References
- MYQORZO™ (aficamten) Prescribing Information (Issued 12/2025).
- SEQUOIA-HCM Clinical Trial Data.
- MYQORZO REMS Program Documentation.
- Aficamten is superior to metoprolol for symptomatic obstructive hypertrophic cardiomyopathy[European Society of Cardiology]