As the pharmaceutical landscape becomes increasingly complex, the US Food and Drug Administration (USFDA) has definitively shifted from a purely reactive, punitive compliance model to a proactive, risk-based oversight framework. This strategic evolution, championed by the principles of ICH Q9 Quality Risk Management (QRM), fundamentally changes how pharmaceutical manufacturers prepare for, and interact with, regulatory inspectors.
This comprehensive guide, authored by a USFDA regulatory expert, synthesizes the core principles of ICH Q9, the nuances of the recent Q9(R1) revisions, and the practical application necessary to achieve and maintain excellence in a risk-based inspection era.
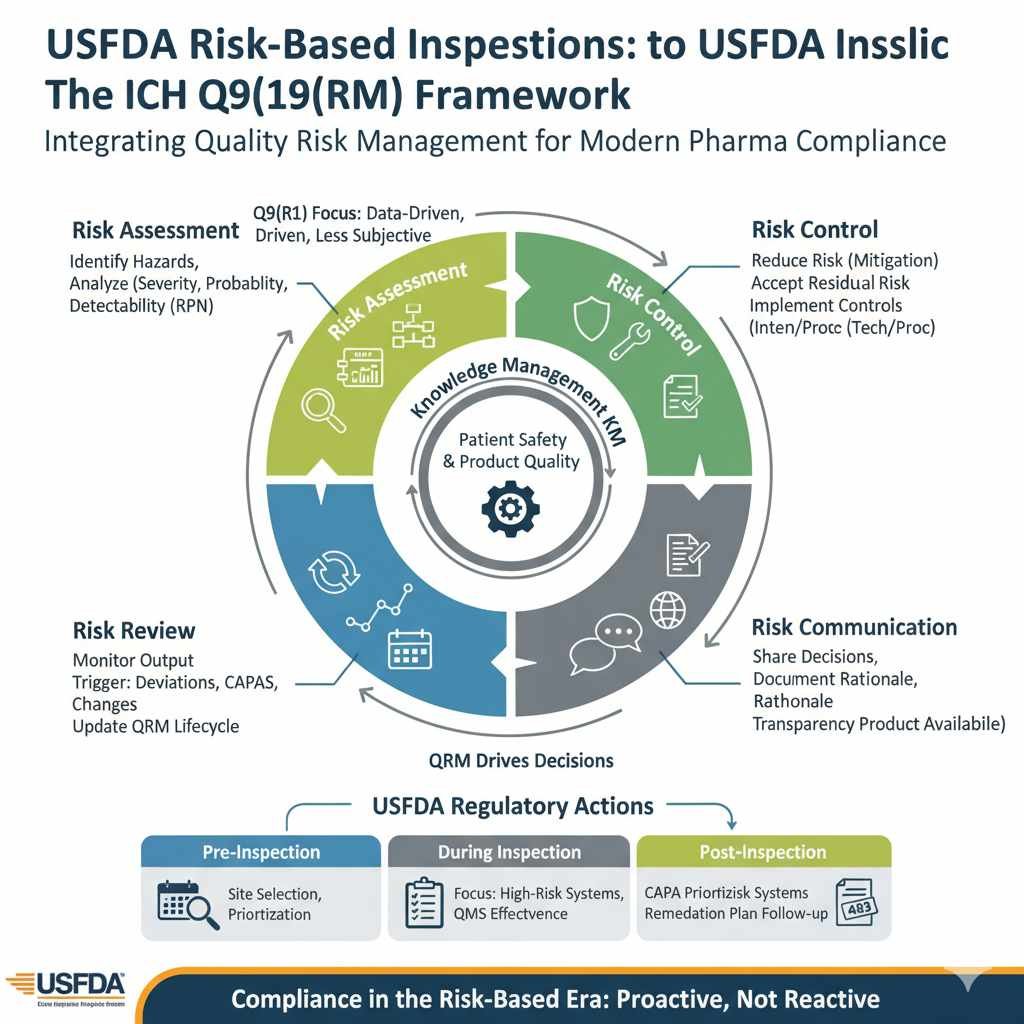
Risk-Based USFDA Inspections ICH Q9 Framework: An Overview
The USFDA’s movement toward risk-based oversight is rooted in the Pharmaceutical Current Good Manufacturing Practice (CGMP) for the 21st Century initiative. The agency recognized that simply auditing adherence to procedures was insufficient; instead, oversight must prioritize systems that pose the greatest risk to product quality and patient safety.
This focus is formalized through several mechanisms that directly align with ICH Q9 principles:
Risk-Based Site Selection and Scheduling
The USFDA uses a sophisticated, data-driven approach to determine who, when, and how frequently a facility is inspected. Key factors in the agency’s risk models (such as the System for Ranking Potential Recalls or Compliance Risk Indicator) include:
- Compliance History: The number and severity of previous Form 483 observations, Warning Letters, or import alerts.
- Product Risk Profile: Facilities manufacturing high-risk products (e.g., sterile injectables, specialized biologics, complex dosage forms) are prioritized.
- Time Since Last Inspection: Regulatory statutes require periodic inspection, but risk determines urgency and frequency.
- Process Complexity: Manufacturing processes involving novel technology or high variability are considered higher risk.
| Risk-Based Principle (ICH Q9) | USFDA Inspection Application |
| Severity and Probability | Inspection findings (e.g., 483 observations) are weighted by the severity of the potential impact on the patient and the probability of occurrence if not corrected. |
| Knowledge Management | FDA expects companies to use internal data (trending, process knowledge) as evidence of effective risk control and proactive management. |
| Resource Allocation | Inspection duration and depth are scaled to the facility’s perceived risk profile. High-risk facilities receive more extensive and specialized inspections. |
ICH Q9 Quality Risk Management (QRM)
ICH Q9, formally titled Quality Risk Management, provides the systematic process for the assessment, control, communication, and review of quality risks throughout the product lifecycle. The FDA recognizes this guideline as the global standard for establishing a robust Quality Risk Management System (QRMS).
The Four Core QRM Components
The guideline establishes a structured, cyclical process that must be applied with rigor and consistency:
| QRM Component | Description | Integration with FDA Inspection Agenda |
| 1. Risk Assessment | Systematic Risk Identification, Analysis (likelihood/severity), and Evaluation (comparing identified risk against acceptable risk levels). | Inspectors examine the scientific basis and comprehensiveness of the risk identification stage (e.g., using Process Mapping or FMEA). |
| 2. Risk Control | Decision-making to reduce risk to an acceptable level or formally document Risk Acceptance of residual risk. | Regulators assess whether the controls implemented (technical or procedural) are sufficient, justified by the initial risk, and effective in practice. |
| 3. Risk Communication | Transparent sharing of risk information and decisions among internal and external stakeholders (including regulators). | Ensures that the rationale for critical decisions (e.g., batch disposition, scope of validation) is traceable, documented, and scientifically sound. |
| 4. Risk Review | Monitoring the output of the QRM process. Risks must be revisited when knowledge changes, new failures occur, or controls degrade. | The system must demonstrate that risk assessments are living documents, triggered for review by deviations, CAPAs, OOS results, or significant change controls. |
Insight into Q9(R1) Revisions: New Regulatory Focus Areas
The 2023/2024 revision to ICH Q9 (Q9(R1)) clarified several areas that were previously prone to misinterpretation or poor implementation, directly tightening regulatory expectations for FDA compliance.
Key Q9(R1) Focus Areas and Compliance Strategies
The FDA views compliance through the lens of Q9(R1)’s strengthened emphasis on subjectivity, formality, and product availability.
A. Degree of Formality (Bridging the Gap: JDDTOnline Insight)
The revision demands that the level of effort, formality, and documentation must be commensurate with the level of risk to quality and patient safety.
- Pitfall: Applying the same exhaustive FMEA process to a minor documentation change as to a complex sterile processing change.
- Compliance Strategy: Define and document a Quality Risk Management Plan that clearly outlines the triggers for Formal QRM (requiring cross-functional teams, established tools like FMEA or HACCP, and stand-alone reports for high uncertainty, importance, or complexity) versus Informal QRM (using simple techniques like flowcharts and documenting the rationale within the Quality System, typically for low-complexity/low-impact issues).
| Factor | High (Formal QRM Required) | Low (Informal QRM Acceptable) |
| Uncertainty | Lack of knowledge about hazards (e.g., new dosage form, complex OOS). | Good knowledge; easily answer ‘what can go wrong’ (e.g., repetitive minor deviation). |
| Importance | High degree of importance relative to product quality (e.g., critical deviation, major change control). | Low degree of importance (e.g., minor change control not impacting product quality). |
| Complexity | Highly complex process (e.g., new manufacturing process). | Low complexity, simple to understand process. |
B. Managing Subjectivity
Q9(R1) stresses the need to minimize the inherent subjectivity in risk scoring. This impacts the reliability of the Risk Priority Number (RPN) calculation (Severity $\times$ Probability $\times$ Detectability).
- Regulatory Interpretation: The FDA will challenge QRM outcomes where the scoring scales (e.g., “Severity 5”) are not clearly defined or are inconsistently applied across different departments or teams.
- Compliance Strategy: Institute clear, defined, and auditable rating criteria for all elements of the RPN. Ensure QRM teams are cross-functional (e.g., Quality, Process Engineering, Regulatory, Production) to pool expertise and mitigate individual bias.
C. Product Availability and Supply Chain
The revision explicitly connects quality risk to potential drug shortages.
- Regulatory Interpretation: Quality failures that lead to manufacturing stoppages and impact the availability of critical medicines must be factored into the QRM scope.
- Compliance Strategy: Integrate supply chain criticality into risk assessments. For example, risk assessments on single-source critical materials or unique manufacturing steps must include the consequence (Severity) of a failure leading to market disruption.
4. QRM Implementation: Achieving FDA Inspection Readiness
A robust QRM program is the ultimate evidence of control and compliance effectiveness. It must be demonstrated throughout the Quality Management System (QMS), supported by Knowledge Management (KM).
The Critical Role of Knowledge Management (KM)
As highlighted by Pharmaceutical Technology, KM is the foundation of objective QRM. Knowledge (historical data, trending, process understanding, development reports) transforms risk assessment from subjective opinion to objective fact.
| KM Input | QRM Output/Use |
| Annual Product Review (APR) Trends | Used to assign the Probability score in RPN based on actual historical failure rates. |
| Post-Approval Change History | Used to identify and re-assess risk for processes that have undergone multiple changes. |
| Deviation and CAPA Effectiveness Data | Used during Risk Review to verify that mitigation actions successfully reduced risk as predicted. |
| Development Studies (QbD) | Provides the scientific basis for determining Severity and defining Critical Quality Attributes (CQAs) and Critical Process Parameters (CPPs). |
Practical QRM Application: Influencing Regulatory Outcomes
QRM should directly influence and prioritize a company’s actions, demonstrating true risk control to an FDA investigator.
| Real-World Application | QRM Influence | Inspection Expectation |
| CAPA Prioritization | CAPAs addressing failures stemming from High-RPN risks must be executed and verified before those addressing low-level risks. | The CAPA system must include a mandatory QRM linkage that drives priority, resource allocation, and timeline setting. |
| Post-Inspection Remediation | When responding to a Form 483 or Warning Letter, QRM justifies the scope and timeline of the remediation plan. | The response must link the proposed CAPA directly back to the original risk assessment, proving the corrective action reduces the residual risk to an acceptable level. |
| Process Validation Scope | QRM determines which process steps are Critical and thus require the most extensive testing, sampling, and documentation during the validation protocol. | Inspectors verify that the firm’s QRM process identified the appropriate CPPs and CQAs, and that validation efforts match the risk level. |
Lessons Learned and Strategies to Avoid Pitfalls
Recent FDA and EMA inspections under the new Q9 focus reveal common pitfalls related to flawed or incomplete QRM implementation:
- “Shelfware” Syndrome: Risk assessments were completed once but never reviewed or updated, rendering them obsolete. Strategy: Integrate formal QRM review into the change control, deviation, and CAPA closure processes.
- Lack of Scientific Justification: Risk scores were assigned arbitrary values (e.g., “Medium”) without objective data (KM). Strategy: Always support Probability and Severity ratings with objective data from the QMS (complaint rates, failure rates, etc.).
- Mismatched Formality: Using Informal QRM (e.g., a simple brainstorming session) to manage a high-risk system (e.g., Data Integrity for electronic records). Strategy: Strictly adhere to the Q9(R1) guidelines on the Degree of Formality; critical systems require the rigor of Formal QRM tools.
- Accepting Excessive Residual Risk: Failing to define the point at which residual risk is unacceptable, or accepting high risk without documented rationale or senior management sign-off. Strategy: Establish a documented Quality Risk Acceptance Policy with defined escalation paths and clear acceptance criteria.
Frequently Asked Questions (FAQs)
How does the FDA evaluate a firm’s QRM culture during an inspection?
Advanced Answer: The FDA assesses QRM culture not by reviewing a single QRM document, but by observing how risk is integrated into daily decision-making. Inspectors look for evidence that decisions on batch disposition, deviation handling, and resource allocation are consistently driven by a risk-based rationale. A key indicator is whether low-level personnel are empowered to stop a process based on an identified risk, and whether senior management provides the necessary resources and time for formal QRM execution, demonstrating the “tone at the top.”
Can QRM justify a reduction in batch testing frequency post-approval?
Advanced Answer: Yes, but only through a rigorous, data-driven Risk Review process that culminates in a controlled change. If extensive KM and successful Ongoing Process Verification (OPV) data prove a process parameter is consistently controlled and robust (low probability of failure), QRM can justify reducing testing frequency (e.g., moving from 100% testing to skipped testing for a non-critical attribute). This justification must be submitted to the FDA as a post-approval change supported by the QRM documentation.
What is the single most common QRM compliance deficiency observed by the FDA?
Advanced Answer: The most common critical deficiency is the failure to adequately perform Risk Review, which encompasses a lack of integration with the CAPA system. Firms often complete a risk assessment but fail to formally revisit and update it when a CAPA is implemented. If the CAPA fails to correct the issue, the risk score is proven inaccurate, yet the original QRM remains unchanged, leaving the true risk level unrecognized and unmitigated—a direct violation of the Q9 core lifecycle principle.
Conclusion: Actionable Recommendations for the Risk-Based Era
The USFDA’s alignment with ICH Q9 principles makes QRM the central component of regulatory compliance. It is the manufacturer’s opportunity to proactively define and control risk, rather than waiting for regulatory scrutiny.
Actionable Recommendations for Maintaining FDA Compliance:
- Define and Audit Formality: Establish clear, written procedures for defining the Degree of Formality (Formal vs. Informal QRM) based on Uncertainty, Importance, and Complexity, and routinely audit these internal decisions.
- Institutionalize Knowledge Management: Mandate the use of KM data (APR, deviation trends, stability reports) as the required input for all Risk Assessment steps to eliminate subjectivity.
- Link CAPA to QRM Lifecycle: Revise procedures so that CAPA Effectiveness Checks trigger a mandatory Risk Review of the original assessment to ensure the risk score and controls are still valid.
- Elevate Risk Communication: Ensure senior management formally reviews and approves all decisions regarding Risk Acceptance, especially those involving high Residual Risk, demonstrating accountability to the FDA.
By embracing QRM not as a checklist, but as a continuous system of scientific and evidence-based decision-making, pharmaceutical manufacturers can transform inspection readiness from a reactive effort into an embedded pillar of product quality.
References
- International Council for Harmonisation (ICH). ICH guideline Q9 (R1) on quality risk management – Step 5 – Revision 2. European Medicines Agency (EMA); 2023(https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use-ich-guideline-q9-r1-quality-risk-management-step-5-revision-2_en.pdf).
- U.S. Food and Drug Administration (FDA). Q9(R1) Quality Risk Management Guidance for Industry. U.S. Department of Health and Human Services; 2023.
- Pathodiya, M. “Quality Risk Management: Degree of formality, Formal and Informal QRM.” Journal of Drug Delivery and Therapeutics. 2025; 15(2):124-128. doi:10.22270/jddt.v15i2.6978. (Content used to elaborate on the relationship between Uncertainty, Importance, Complexity, and Degree of Formality).
- O’Donnell, K. “QRM, Knowledge Management, and the Importance of ICH Q9(R1).” Pharmaceutical Technology. July 8, 2024. (Content used to establish the necessity of Knowledge Management as the foundation for objective QRM).[https://www.pharmtech.com/view/qrm-knowledge-management-and-the-importance-of-ich-q9-r1-]
- U.S. Food and Drug Administration (FDA). Overview of Changes: ICH Q9 (R1) Quality Risk Management. [Presentation/Training Material]. FDA; May 2023. (Used for detailed revision topics including Subjectivity, Product Availability, and Formality as inspection focus areas).[https://www.fda.gov/media/177720/download]
- Redica Systems. “ICH Q9: Quality Risk Management Revision Focuses on Six Topic Areas.” Redica Systems Blog. February 3, 2022. (Content used for outlining the key focus areas driving the Q9(R1) revision and regulatory goals for risk-based oversight).
- ICH. ICH guideline Q9 on quality risk management – Step 5 – First version. European Medicines Agency (EMA); 2006. (Used for core concepts: Risk Assessment, Control, Communication, and Review).
- FDA. Pharmaceutical Current Good Manufacturing Practice (CGMP) for the 21st Century — A Risk-Based Approach. FDA Initiative, 2004. (Used to establish the historical context for the FDA’s shift to a risk-based inspection framework).
- ICH. ICH Q10 Pharmaceutical Quality System. ICH; 2008. (Cited to support the role of QRM and Knowledge Management as enabling elements within a Pharmaceutical Quality System).



