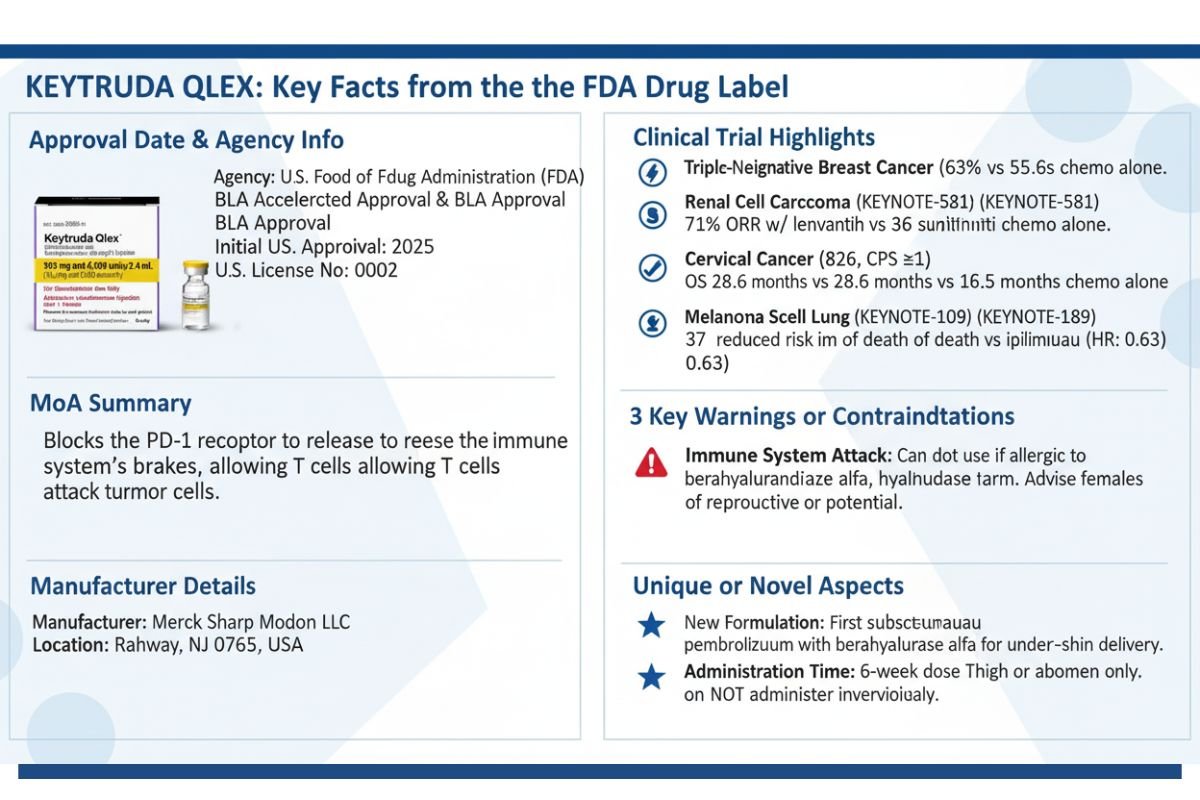
In a significant development for oncology practice, the U.S. Food and Drug Administration (FDA) on September 19, 2025, granted approval for Keytruda Qlex (pembrolizumab and berahyaluronidase alfa-pmph), a new subcutaneous formulation of the widely used PD-1 inhibitor, pembrolizumab. This approval introduces a more convenient administration option for patients and healthcare systems, shifting from intravenous infusion to a rapid subcutaneous injection.
Developed by Merck Sharp & Dohme LLC, Keytruda Qlex combines pembrolizumab with berahyaluronidase alfa, an enzyme that facilitates under-the-skin delivery. The approval covers an extensive range of indications across numerous solid tumors—including melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), and triple-negative breast cancer (TNBC)—mirroring the approvals of its intravenous predecessor.
This new formulation was approved based on data demonstrating a comparable pharmacokinetic, efficacy, and safety profile to intravenous pembrolizumab, marking a new chapter in the administration of cancer immunotherapy. This article provides a comprehensive overview for healthcare professionals on the mechanism, clinical data, administration protocols, and safety profile of this novel formulation.
What is Keytruda Qlex?
The approval of Keytruda Qlex represents a pivotal advancement in the administration of one of the cornerstones of modern immuno-oncology. For years, clinicians have administered pembrolizumab via intravenous infusion, a process that requires significant time and healthcare resources. This new formulation addresses these logistical challenges directly by enabling rapid subcutaneous delivery, potentially enhancing the patient experience and optimizing clinic workflow.
Composition and Innovative Formulation
Keytruda Qlex is a sterile, preservative-free, ready-to-use solution provided as a fixed-dose combination product. It contains two active ingredients:
- Pembrolizumab: The well-established humanized monoclonal IgG4 kappa antibody that targets the PD-1 receptor.
- Berahyaluronidase alfa-pmph: A novel endoglycosidase enzyme. Its function is to enhance the dispersion and absorption of the co-administered pembrolizumab in the subcutaneous space.
This dual-component formulation is the key to its innovation. The subcutaneous tissue space typically cannot accommodate the large volumes required for a full therapeutic dose of a monoclonal antibody like pembrolizumab. By incorporating berahyaluronidase alfa, the formulation temporarily modifies the subcutaneous environment to allow for the administration of a larger volume, making subcutaneous delivery feasible without compromising the dosage. The product is available in two configurations to support different dosing schedules: a 395 mg/4,800 unit vial and a 790 mg/9,600 unit vial.
The Rationale for Subcutaneous Delivery
The transition from intravenous to subcutaneous administration for systemic cancer therapies is a significant trend aimed at improving both patient care and healthcare system efficiency. Intravenous infusions necessitate venous access, prolonged chair time in an infusion center, and the associated personnel and facility resources. A subcutaneous injection, in contrast, can be administered in a matter of minutes.
For patients, this can mean a substantial reduction in the time spent in the clinic, less discomfort associated with IV lines, and a less burdensome treatment experience overall. For healthcare providers in busy oncology practices across the U.S. and India, this formulation can help optimize patient scheduling, reduce infusion chair wait times, and potentially allow for the treatment of more patients. The approval letter and prescribing information for Keytruda Qlex underscore that this formulation has different administration instructions than its intravenous counterpart, and the two should not be used interchangeably, highlighting the importance of clinical vigilance during this transition.
Unraveling the Dual Mechanism of Action
The therapeutic effect of Keytruda Qlex is driven by a synergistic combination of two distinct mechanisms of action: the immunotherapeutic activity of pembrolizumab and the delivery-enabling function of berahyaluronidase alfa.
Pembrolizumab: Releasing the Brakes on the Immune System
Pembrolizumab’s mechanism is central to the field of cancer immunotherapy. Its target is the programmed death receptor-1 (PD-1), a crucial checkpoint receptor found on the surface of activated T cells. In a healthy state, the PD-1 pathway helps maintain immune homeostasis and prevent autoimmunity by downregulating T-cell activity when the receptor binds to its ligands, PD-L1 and PD-L2.
Many tumor cells exploit this natural regulatory pathway to evade immune destruction. They upregulate the expression of PD-L1 on their surface, and when these ligands engage with the PD-1 receptor on tumor-infiltrating T cells, the T cells become anergic or exhausted, effectively shutting down the anti-tumor immune response.
Pembrolizumab is a high-affinity monoclonal antibody that physically binds to the PD-1 receptor and blocks it from interacting with both PD-L1 and PD-L2. This blockade cuts the inhibitory signal from the tumor cell to the T cell. By “releasing the brakes,” pembrolizumab restores the T cell’s ability to recognize and attack cancer cells, leading to a potent, targeted anti-tumor immune response. This mechanism is not specific to one tumor type but can be effective across any cancer that relies on the PD-1/PD-L1 pathway for immune evasion, which explains its broad range of approved indications .
Berahyaluronidase Alfa: The Key to Subcutaneous Administration
The primary challenge of administering large-volume biologics like pembrolizumab subcutaneously is the physical resistance of the extracellular matrix in the subcutaneous tissue. This matrix is rich in hyaluronan, a polysaccharide that limits the dispersion of large fluid volumes.
Berahyaluronidase alfa is a recombinant form of a naturally occurring human enzyme, hyaluronidase PH20, that specifically degrades hyaluronan. When injected into the subcutaneous space as part of the Keytruda Qlex formulation, it rapidly and temporarily breaks down the local hyaluronan network. This action decreases the viscosity of the interstitial space and increases the hydraulic conductivity, thereby enhancing the permeability of the tissue. This allows the co-administered volume of pembrolizumab solution to disperse over a wider area and be absorbed more effectively into systemic circulation.
Crucially, this enzymatic effect is localized and fully reversible. The body naturally replenishes the hyaluronan, and the permeability of the subcutaneous tissue is restored to its normal state within 24 to 48 hours. This transient mechanism enables the successful subcutaneous delivery of a full therapeutic dose of pembrolizumab without the need for an IV line.
Broad Therapeutic Spectrum: Approved Indications for Keytruda Qlex
Keytruda Qlex is approved for all indications previously held by the intravenous formulation of pembrolizumab, underscoring the FDA’s confidence in its comparable efficacy and safety profile. These indications span a wide array of solid tumors, both as a monotherapy and as part of combination regimens. Patient selection for certain indications is dependent on biomarker status, such as PD-L1 expression, microsatellite instability-high (MSI-H), or tumor mutational burden-high (TMB-H).
Traditional Approval Indications
- Melanoma: For the treatment of unresectable or metastatic melanoma and for the adjuvant treatment of adult and pediatric patients (12 years and older) with Stage IIB, IIC, or III melanoma following complete resection .
- Non-Small Cell Lung Cancer (NSCLC): Approved for multiple settings in NSCLC, including first-line treatment of metastatic disease (squamous and nonsquamous) in combination with chemotherapy; as a single agent for first-line treatment of PD-L1-positive tumors; for previously treated metastatic NSCLC; and for the neoadjuvant/adjuvant treatment of resectable NSCLC .
- Head and Neck Squamous Cell Cancer (HNSCC): For the first-line treatment of metastatic or unresectable, recurrent HNSCC, both as a single agent (for PD-L1 positive tumors) and in combination with chemotherapy, as well as for previously treated patients .
- Urothelial Carcinoma: For use in combination with enfortumab vedotin for locally advanced or metastatic disease, and as a single agent for patients who are not eligible for or have progressed on platinum-containing chemotherapy . It is also approved for certain patients with high-risk, non-muscle invasive bladder cancer (NMIBC).
- MSI-H or dMMR Cancers: For adult and pediatric patients (12 years and older) with unresectable or metastatic MSI-H or mismatch repair deficient (dMMR) solid tumors that have progressed following prior treatment, and for patients with MSI-H or dMMR colorectal cancer .
- Gastric and Esophageal Cancers: Approved for the first-line treatment of HER2-positive and HER2-negative gastric or gastroesophageal junction (GEJ) adenocarcinoma (for PD-L1 positive tumors) in combination with chemotherapy, and for locally advanced or metastatic esophageal or GEJ carcinoma .
- Cervical Cancer: Approved in combination with chemoradiotherapy for locally advanced disease, and in combination with chemotherapy (with or without bevacizumab) or as a single agent for persistent, recurrent, or metastatic disease in patients with PD-L1 positive tumors .
- Other Solid Tumors: The drug also holds approvals for Malignant Pleural Mesothelioma (MPM), Hepatocellular Carcinoma (HCC), Biliary Tract Cancer (BTC), Merkel Cell Carcinoma (MCC), Renal Cell Carcinoma (RCC), Endometrial Carcinoma, Cutaneous Squamous Cell Carcinoma (cSCC), and Triple-Negative Breast Cancer (TNBC) in various settings .
Accelerated Approval Indication
- Tumor Mutational Burden-High (TMB-H) Cancer: Keytruda Qlex received accelerated approval for treating adult and pediatric patients (12 years and older) with unresectable or metastatic TMB-H [≥10 mutations/megabase (mut/Mb)] solid tumors that have progressed on prior therapy and have no other satisfactory treatment options. Continued approval for this indication is contingent upon verification of clinical benefit in confirmatory trials.
Clinical Efficacy and Pivotal Trial Data
The comprehensive approval of Keytruda Qlex hinges on two pillars of evidence: a pivotal bridging study demonstrating the comparability of the subcutaneous formulation to the intravenous formulation, and the extensive body of efficacy data from the foundational KEYNOTE clinical trial program for intravenous pembrolizumab.
The Bridge to Subcutaneous Approval: The MK-3475A-D77 Study
The pivotal study supporting this new formulation was MK-3475A-D77, a randomized, open-label trial in 377 patients with previously untreated metastatic NSCLC. The trial was designed not to re-establish efficacy in NSCLC, but to demonstrate that the subcutaneous formulation delivered a comparable amount of the drug into the bloodstream and produced similar clinical outcomes as the intravenous version.
- Primary Outcome: The study successfully met its primary endpoints for pharmacokinetics. It demonstrated that the drug exposure at Cycle 1 (AUC0-6wks) and the trough concentration at steady state (Cycle 3 Ctrough) for subcutaneous Keytruda Qlex were comparable to intravenous pembrolizumab.
- Efficacy and Safety: Clinical outcomes were consistent between the two arms. The confirmed Overall Response Rate (ORR) was 45% in the subcutaneous Keytruda Qlex arm, compared to 42% in the intravenous pembrolizumab arm. The safety profiles were also similar, establishing that the subcutaneous formulation did not introduce new or unexpected safety concerns.
Foundation of Efficacy: Key Data from Intravenous Pembrolizumab Trials
With comparability established, the efficacy of Keytruda Qlex for its broad range of indications rests on the robust data from the extensive KEYNOTE clinical trial program for intravenous pembrolizumab. A few highlights from this program include:
- Melanoma (KEYNOTE-006): In this trial, intravenous pembrolizumab demonstrated statistically significant improvements in both Overall Survival (OS) and Progression-Free Survival (PFS) compared to ipilimumab in patients with advanced melanoma. The hazard ratio for OS was 0.63, representing a 37% reduction in the risk of death.
- NSCLC (KEYNOTE-189): For the first-line treatment of metastatic nonsquamous NSCLC, adding intravenous pembrolizumab to standard chemotherapy resulted in a significantly longer OS compared to chemotherapy alone (Hazard Ratio: 0.49). The median OS was not reached in the pembrolizumab arm, versus 11.3 months in the chemotherapy arm at the time of the primary analysis.
- Triple-Negative Breast Cancer (KEYNOTE-522): In the neoadjuvant and adjuvant setting for high-risk early-stage TNBC, adding pembrolizumab to chemotherapy significantly increased the rate of pathological complete response (pCR) to 63% from 55.6% with chemotherapy alone. The regimen also demonstrated a statistically significant improvement in event-free survival (EFS), with a hazard ratio of 0.63.
- Renal Cell Carcinoma (KEYNOTE-581): As a first-line treatment for advanced RCC, the combination of intravenous pembrolizumab and lenvatinib showed superior OS, PFS, and ORR compared to sunitinib. The ORR was 71% for the combination arm versus 36% for the sunitinib arm.
Dosage and Administration Guidelines for Clinicians
The administration of Keytruda Qlex is fundamentally different from that of intravenous pembrolizumab. It is critical for healthcare teams to be familiar with the new protocols to ensure patient safety and prevent medication errors.
Recommended Dosing Regimens
Keytruda Qlex offers two convenient dosing schedules for adults and pediatric patients (12 years and older weighing more than 40 kg):
- Every 3 Weeks: One 395 mg/4,800 unit (2.4 mL) vial is administered as a subcutaneous injection over approximately 1 minute.
- Every 6 Weeks: One 790 mg/9,600 unit (4.8 mL) vial is administered as a subcutaneous injection over approximately 2 minutes.
The choice of regimen and the duration of treatment depend on the specific indication and are detailed in the full prescribing information.
Preparation and Administration Protocol
- Administration: Keytruda Qlex must be administered by a healthcare provider. It is for subcutaneous use only and should never be administered intravenously.
- Injection Site: The injection should be delivered into the thigh or abdomen, avoiding the 5 cm area around the navel. Injection sites should be rotated and should not be into skin that is red, bruised, tender, or hard.
- Preparation: The vial should be brought to room temperature for at least 30 minutes before use. The solution is ready to use and should not be diluted or shaken. A sterile syringe and an 18- to 21-gauge transfer needle should be used to withdraw the correct volume from the vial. The needle must be changed to a 25- to 30-gauge injection needle immediately prior to administration.
- Storage: Vials must be stored refrigerated at 2°C to 8°C (36°F to 46°F) and protected from light. The prepared syringe can be stored at room temperature for up to 8 hours or refrigerated for up to 24 hours if not used immediately.
Comprehensive Safety Profile: Warnings and Adverse Events
The safety profile of Keytruda Qlex is consistent with that of intravenous pembrolizumab. The prescribing information carries significant warnings regarding immune-mediated adverse reactions, which require diligent monitoring and management.
Key Warnings and Precautions
- Severe and Fatal Immune-Mediated Adverse Reactions: This is the most critical warning. Because pembrolizumab enhances the immune system, it can cause the immune system to attack healthy organs and tissues, leading to severe or fatal inflammatory reactions. These can occur at any time during or after treatment and may affect any organ system. Clinicians must monitor for:
- Pneumonitis: New or worsening cough, chest pain, or shortness of breath.
- Colitis: Diarrhea or severe abdominal pain.
- Hepatitis: Jaundice, severe nausea or vomiting, or easy bruising.
- Endocrinopathies: Adrenal insufficiency, hypophysitis, thyroid disorders, and type 1 diabetes mellitus.
- Nephritis with renal dysfunction and severe dermatologic reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN).Management generally involves withholding or discontinuing Keytruda Qlex and administering systemic corticosteroids.
- Hypersensitivity and Administration-Related Reactions: Severe or life-threatening reactions, including anaphylaxis, can occur.
- Complications of Allogeneic HSCT: Patients who receive an allogeneic hematopoietic stem cell transplant before or after treatment with a PD-1 inhibitor are at risk for serious complications, including graft-versus-host-disease (GVHD).
- Embryo-Fetal Toxicity: Keytruda Qlex can cause fetal harm. Females of reproductive potential must be advised of the risk and use effective contraception during treatment and for 4 months after the final dose.
Common Adverse Reactions
The most common adverse reactions (
≥20%) observed in the pivotal trial of Keytruda Qlex in combination with chemotherapy were nausea (25%), fatigue (25%), and musculoskeletal pain (21%).
The broader safety profile, based on the extensive experience with intravenous pembrolizumab, includes the following common adverse reactions:
- As Monotherapy: Fatigue, musculoskeletal pain, rash, diarrhea, pyrexia, cough, decreased appetite, pruritus, dyspnea, constipation, nausea, and hypothyroidism.
- In Combination with Chemotherapy: Fatigue/asthenia, nausea, constipation, diarrhea, decreased appetite, rash, vomiting, cough, alopecia, and peripheral neuropathy.
- In Combination with Axitinib or Lenvatinib: Diarrhea, hypertension, hypothyroidism, hepatotoxicity, and palmar-plantar erythrodysesthesia are prominent.
Pharmacokinetic and Pharmacodynamic Profile
- Pharmacokinetics: Following subcutaneous administration, pembrolizumab has a bioavailability of approximately 60%. Peak concentrations are reached in about 4 days. It has a volume of distribution of 6.0 L and a terminal half-life of 22 days. Steady state is reached by 16 weeks. The pharmacokinetics are not significantly affected by age, race, sex, body weight, tumor type, or mild to moderate renal or hepatic impairment.
- Pharmacodynamics: The exposure levels achieved with the approved subcutaneous dosing regimens of Keytruda Qlex fall within the range of exposures observed with the effective intravenous regimens. There are no clinically significant exposure-response relationships for the efficacy or safety of pembrolizumab across its approved doses.
The Regulatory Journey
The approval of Keytruda Qlex was managed through a Biologics License Application (BLA 761467) that was split into two separate actions by the FDA:
- BLA 761467/Original 1: Covered the extensive list of indications receiving traditional approval.
- BLA 761467/Original 2: Covered the single indication for TMB-H cancers, which was granted under the FDA’s Accelerated Approval pathway.
The application was submitted on January 23, 2025, and received approval on September 19, 2025. No FDA advisory committee meeting was convened, as the agency determined that outside expertise was not necessary and there were no controversial issues that would benefit from such a discussion. For the accelerated approval, Merck is required to conduct postmarketing trials to verify and describe the clinical benefit for the TMB-H indication, with a final report due by December 2025.
Frequently Asked Questions (FAQs)
Can a patient currently receiving intravenous pembrolizumab be switched to subcutaneous Keytruda Qlex?
Yes, The prescribing information states that patients receiving intravenous pembrolizumab can switch to subcutaneous Keytruda Qlex at their next scheduled dose, and vice-versa
What is the precise role of the berahyaluronidase alfa component in Keytruda Qlex?
Berahyaluronidase alfa is an enzyme that temporarily breaks down hyaluronan, a substance in the subcutaneous tissue. This increases the tissue’s permeability, allowing the large fluid volume of the pembrolizumab dose to be administered and absorbed effectively from under the skin.
How should a clinician manage a suspected immune-mediated adverse reaction?
For severe (Grade 3) immune-mediated adverse reactions, Keytruda Qlex should generally be withheld. For life-threatening (Grade 4) reactions, it should be permanently discontinued. Systemic corticosteroid therapy (e.g., prednisone 1-2 mg/kg/day) should be administered until the reaction improves to Grade 1 or less, followed by a corticosteroid taper over at least one month.
Are there any differences in the safety profile between the 3-week and 6-week dosing regimens of Keytruda Qlex?
The prescribing information does not indicate a difference in the safety profile between the 3-week and 6-week dosing regimens. The 6-week dose (790 mg) provides drug exposure levels that are within the range of the effective intravenous regimens, similar to the 3-week dose (395 mg).
Does Keytruda Qlex need to be diluted before administration?
No. Keytruda Qlex is a ready-to-use solution and must not be diluted. It should be withdrawn directly from the vial into a syringe for administration.
References
- U.S. Food and Drug Administration. BLA APPROVAL Letter for BLA 761467/Original 1. September 19, 2025.
- U.S. Food and Drug Administration. BLA ACCELERATED APPROVAL Letter for BLA 761467/Original 2. September 19, 2025.
- KEYTRUDA QLEX™ (pembrolizumab and berahyaluronidase alfa-pmph) injection, for subcutaneous use. Prescribing Information. Merck Sharp & Dohme LLC. Revised: 09/2025.
- ClinicalTrials.gov. A Study of Subcutaneous Pembrolizumab Versus Intravenous Pembrolizumab in Participants With Non-small Cell Lung Cancer (PALLAS-2/KEYNOTE-D77). Identifier: NCT05722015.
- Garon, EB, et al. “Pembrolizumab for the treatment of non–small-cell lung cancer.” The New England Journal of Medicine 372.21 (2015): 2018-2028.
- O’Donnell, JS, et al. “The dawn of T-cell-directed immunotherapy.” The Lancet 398.10300 (2021): 637-652.
- Gilbert, A, et al. “Pharmacokinetics of Pembrolizumab After Subcutaneous Administration in Patients With Melanoma.” Clinical Pharmacology & Therapeutics 110.3 (2021): 793-801.
- Gandhi, L, et al. “Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer.” The New England Journal of Medicine 378.22 (2018): 2078-2092.