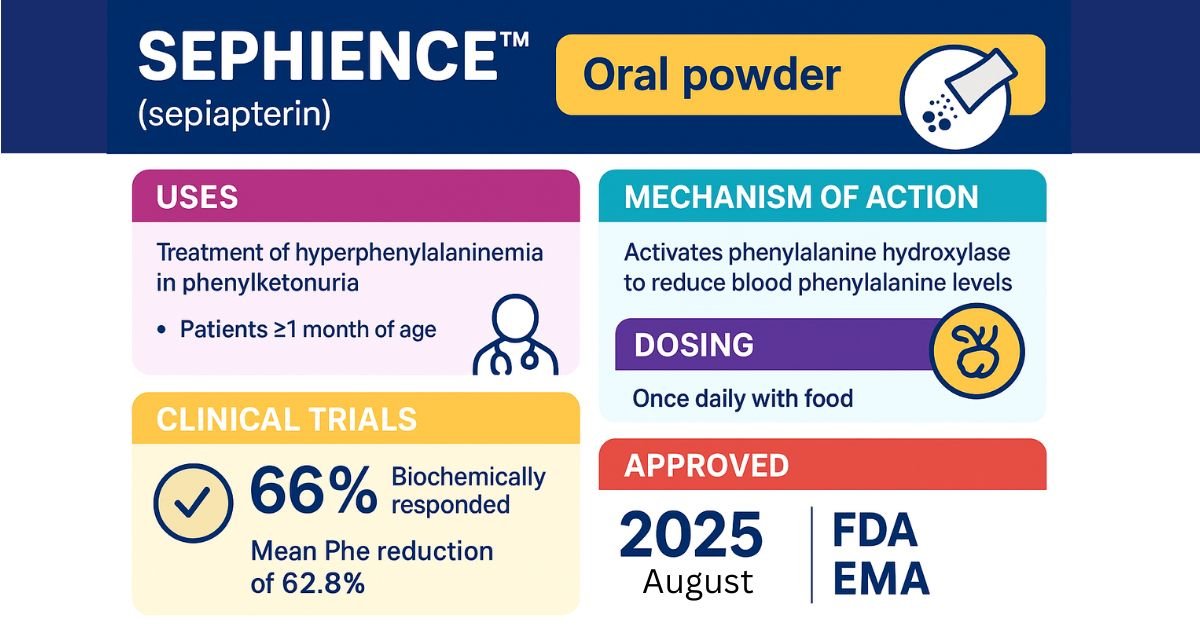
SEPHIENCE (sepiapterin) is a novel phenylalanine hydroxylase (PAH) activator that has received approval for treating hyperphenylalaninemia (HPA) in adult and pediatric patients (1 month and older) with sepiapterin-responsive Phenylketonuria (PKU). Patients take SEPHIENCE as a once-daily oral powder with food to significantly lower blood phenylalanine (Phe) levels, a critical goal in preventing the severe neurological damage associated with PKU. This article provides a comprehensive overview for healthcare professionals, covering its mechanism of action, pivotal clinical trial data, detailed dosage and administration guidelines, safety profile, and regulatory status in the US and EU.
Important Takeaways for the Busy Clinician:
- Drug: SEPHIENCE (sepiapterin), an oral PAH activator.
- Indication: For sepiapterin-responsive PKU in patients aged 1 month and older, serving as an adjunct to a Phe-restricted diet.
- Mechanism: As a precursor to the essential cofactor tetrahydrobiopterin (BH4), SEPHIENCE enhances the activity of the deficient PAH enzyme through both cofactor replenishment and chaperone-like stabilization.
- Efficacy: A pivotal placebo-controlled trial showed that SEPHIENCE achieved a mean 62.8% reduction in blood Phe levels from baseline, compared to a 1.4% increase with placebo. The therapy has also demonstrated efficacy in patients who were non-responsive to sapropterin.
- Administration: Patients take the once-daily oral powder with food to maximize absorption. The dosage is weight-based and varies by age.
- Safety: The most common adverse reactions include gastrointestinal issues (diarrhea, abdominal pain), headache, and feces discoloration. Key warnings include an increased bleeding risk, the potential for hypophenylalaninemia (especially in pediatrics), and an interaction with levodopa.
SEPHIENCE (Sepiapterin): A Deep Dive into the New-Age PAH Activator for Phenylketonuria (PKU) Management
The management of Phenylketonuria (PKU) presents a significant clinical challenge, demanding lifelong vigilance and strict dietary adherence to prevent irreversible neurological damage. This rare inherited metabolic disorder places a substantial burden on patients and their families. The recent emergence of SEPHIENCE (sepiapterin) heralds a new era in the therapeutic landscape for PKU, offering a potent pharmacological intervention that targets the core enzymatic deficiency. As a next-generation phenylalanine hydroxylase (PAH) activator, SEPHIENCE has demonstrated remarkable efficacy in reducing blood phenylalanine levels, providing new hope for better disease control and an improved quality of life.
This article offers a comprehensive, evidence-based overview of SEPHIENCE, tailored for healthcare professionals in the United States and India. We will delve into its unique mechanism of action, analyze the robust data from its clinical trial program, provide practical guidance on dosage and administration, and discuss its safety profile and regulatory journey, including its approvals by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
Understanding the Therapeutic Need in Phenylketonuria (PKU)
The Pathophysiology of PKU
Phenylketonuria is an autosomal recessive inborn error of metabolism stemming from mutations in the
PAH gene, which causes a deficiency in the phenylalanine hydroxylase enzyme. This enzyme is critical for converting the essential amino acid phenylalanine into tyrosine. In its absence, phenylalanine accumulates to toxic levels in the blood and, most critically, in the brain.
If left untreated, this hyperphenylalaninemia (HPA) results in severe clinical sequelae, including profound intellectual disability, epilepsy, behavioral problems, and other devastating neurological complications. For decades, the cornerstone of PKU management has been a highly restrictive, lifelong diet low in phenylalanine. While this diet is effective in preventing the worst outcomes, patients find it notoriously difficult to maintain, which impacts social functioning, nutritional status, and overall quality of life. The development of therapies that can augment or replace strict dietary control is therefore a paramount goal in the field.
The Role of Newborn Screening in the US and India
Early diagnosis is the key to preventing the severe consequences of PKU. In the United States, universal newborn screening (NBS) for PKU is a public health triumph, allowing for the initiation of treatment within the first few days of life. This practice has virtually eliminated the severe intellectual disability once synonymous with the condition.
In India, the landscape is evolving. While NBS programs are established in several states and major metropolitan hospitals, universal implementation is not yet a reality. This disparity means that clinicians in India must maintain a high index of suspicion for metabolic disorders like PKU in infants presenting with developmental delay, seizures, or a distinctive “mousy” odor. The availability of advanced therapies like SEPHIENCE further underscores the importance of expanding NBS programs across the subcontinent to ensure every child has the opportunity for early diagnosis and effective treatment.
SEPHIENCE (Sepiapterin): A Detailed Drug Profile
Mechanism of Action
SEPHIENCE acts as a phenylalanine hydroxylase (PAH) activator. Its therapeutic effect stems from its identity as a precursor to tetrahydrobiopterin (BH4), an indispensable cofactor for the PAH enzyme. However, its mechanism extends beyond simple cofactor replenishment, offering a more sophisticated, dual-action approach.
The mechanism of SEPHIENCE includes:
- Cofactor Replenishment: The body readily metabolizes sepiapterin to form BH4. By increasing the intracellular pool of this essential cofactor, SEPHIENCE helps to drive the residual activity of the deficient PAH enzyme, facilitating the conversion of Phe to tyrosine.
- Pharmacological Chaperone Activity: Beyond serving as a precursor, sepiapterin also acts as a pharmacological chaperone. It can directly bind to and stabilize the misfolded PAH enzyme, helping it achieve a more functional conformation. This stabilization increases the enzyme’s activity and its responsiveness to the available BH4.
This dual mechanism may explain a key clinical finding: SEPHIENCE has demonstrated efficacy in patients who were known to be non-responsive to treatment with sapropterin, an earlier synthetic form of BH4. This suggests that its chaperone-like activity provides a distinct therapeutic advantage, potentially expanding the pool of patients who can benefit from pharmacological therapy.
Evidence from Clinical Trials: Efficacy and Outcomes
A robust clinical development program supports the approval of SEPHIENCE, led by the pivotal Trial 1 (NCT05099640). The study’s design meticulously identified responsive patients and then confirmed the drug’s efficacy against a placebo.
The Pivotal Trial 1 (NCT05099640) Design
This study consisted of two parts and enrolled a broad population of adult and pediatric PKU patients, with ages ranging from 1 to 61 years.
- Part 1 (Open-Label Screening): This initial 14-day phase identified a “sepiapterin-responsive” population. A total of 157 patients received open-label SEPHIENCE.
- Part 2 (Placebo-Controlled, Double-Blind Withdrawal): Patients who demonstrated a significant response in Part 1 were then eligible to enter the second phase. In this 6-week period, 98 patients were randomized to either continue on SEPHIENCE (with doses titrated up to 60 mg/kg/day) or switch to a placebo. This design strongly demonstrates the drug’s specific effect by showing that withdrawal leads to a loss of efficacy.
Key Efficacy Findings
Trial 1 produced statistically significant and clinically meaningful results.
- High Response Rate in Screening: In Part 1, an impressive 66% of patients met the primary response criterion, achieving a reduction in blood Phe levels of 30% or greater. This established a large pool of patients who could derive substantial benefit from the therapy.
- Superiority in Placebo-Controlled Phase: The primary efficacy endpoint measured the mean change in blood Phe from baseline to the final two weeks (Weeks 5 and 6) of Part 2. The results demonstrated a dramatic and clear superiority of SEPHIENCE over placebo:
- SEPHIENCE Group: Patients continuing on the drug saw their mean blood Phe levels plummet by 62.8% from a baseline of 646.1 µmol/L to 236 µmol/L.
- Placebo Group: Patients who switched to placebo saw their Phe levels rebound to their pre-treatment baseline, with their mean levels showing a 1.4% increase from a baseline of 654 µmol/L to 637.9 µmol/L.
- The adjusted mean treatment difference was -395.9 µmol/L, a highly significant outcome confirming that the observed effect was directly attributable to SEPHIENCE.
Supportive Data and Special Populations
Further confidence in SEPHIENCE comes from supportive data from an ongoing open-label study, Trial 2. This trial has shown that the benefits of treatment are maintained over the long term and, critically, that many patients can liberalize their highly restrictive diets—a major improvement in quality of life.
Furthermore, data from these trials have demonstrated efficacy in the crucial pediatric population, including those under 2 years of age. Achieving Phe control during these early years is paramount for protecting neurodevelopment and ensuring optimal cognitive outcomes.
Dosage and Administration
Successful implementation of SEPHIENCE therapy requires a thorough understanding of its dosing, preparation, and monitoring requirements. Physicians with deep knowledge in PKU management must initiate and supervise all treatment.
Initiating Therapy and Dosing Regimen
- Administration: Patients take SEPHIENCE orally once daily and must take it with food. As discussed in the pharmacokinetics section, co-administration with food dramatically increases the bioavailability of the active metabolite.
- Starting Dosage: The recommended starting dose is age- and weight-based:
| Age | Starting Daily Dosage |
| Less than 6 months | 7.5 mg/kg |
| 6 months to <1 year | 15 mg/kg |
| 1 year to <2 years | 30 mg/kg |
| 2 years and older | 60 mg/kg | - Maximum Dose: The maximum recommended daily dose for all patients is 60 mg/kg.
Preparation and Administration Instructions
The manufacturer supplies the drug as an oral powder that must be mixed before administration. The instructions vary based on the total dose.
- For Doses Less Than 1,000 mg:
- Mix the powder with water or apple juice to create a liquid mixture with a final concentration of 25 mg/mL.
- Use a graduated oral dosing syringe to draw up and administer the exact prescribed volume to ensure accurate dosing.
- For Doses 1,000 mg or Greater:
- Mix the entire contents of the required packets with a specified volume of water, apple juice, strawberry jam, or applesauce.
- The patient must consume the entire mixture immediately to ensure they receive the full dose.
The patient should consume additional food after administration of either preparation.
Monitoring and Dose Adjustment
- Response Evaluation: Conduct an initial therapeutic evaluation within the first 2-4 weeks to assess biochemical response.
- Discontinuation Criteria: Discontinue treatment in patients who do not show a decrease in blood Phe after 2 weeks of treatment at the maximum daily dose of 60 mg/kg.
- Ongoing Monitoring: Regular and frequent monitoring of blood Phe levels is essential throughout treatment. This is especially critical in the pediatric population to prevent both hyperphenylalaninemia and iatrogenic hypophenylalaninemia. Based on these levels, clinicians may need to adjust the SEPHIENCE dose and/or the patient’s dietary Phe intake.
Safety, Tolerability, and Risk Management
The SEPHIENCE clinical development program has established a well-defined safety profile. While patients generally tolerate it well, clinicians should remain aware of several key warnings and potential adverse events.
Adverse Events Profile
The prescribing information lists no contraindications for the use of SEPHIENCE.
The most common adverse reactions reported in clinical trials at a higher incidence than placebo included:
- Diarrhea (7% vs. 2% in placebo)
- Headache (7% vs. 2%)
- Abdominal pain (5% vs. 2%)
- Hypophenylalaninemia (4% vs. 0%)
- Feces discoloration (4% vs. 0%)
- Oropharyngeal pain (4% vs. 2%)
Clinicians should counsel patients and caregivers that a yellow or orange discoloration of feces is an expected and benign side effect due to the color of the drug powder.
Key Warnings and Precautions for Clinical Practice
- Increased Bleeding Risk: SEPHIENCE may increase the risk of bleeding. Events including superficial hematomas, prolonged bleeding, and heavy menstrual bleeding were reported in clinical trials. Clinicians should inform patients of this risk and consider treatment interruption if active bleeding occurs.
- Hypophenylalaninemia: This is a particularly important consideration in pediatric patients. Over-correction of Phe levels can lead to hypophenylalaninemia, which, if prolonged, is associated with catabolism and adverse developmental outcomes. This underscores the necessity of frequent and vigilant blood Phe monitoring to maintain levels within the target therapeutic range.
- Interaction with Levodopa: Post-marketing reports with another PAH activator have noted neurological events, including seizures and irritability, during co-administration with levodopa. As SEPHIENCE may increase the availability of tyrosine (a precursor to levodopa), clinicians should closely monitor patients receiving both therapies for any changes in their neurological status.
Significant Drug Interactions
- DHFR and SR Inhibitors: Avoid concomitant use with inhibitors of dihydrofolate reductase (e.g., methotrexate, trimethoprim) or sepiapterin reductase (e.g., sulfasalazine). These drugs may interfere with the metabolic conversion of sepiapterin to its active BH4 form, potentially reducing efficacy. If co-administration is unavoidable, monitor Phe levels more frequently.
- PDE-5 Inhibitors: Both SEPHIENCE and PDE-5 inhibitors (e.g., sildenafil) can induce vasorelaxation. Concomitant use may lead to an additive effect on blood pressure. Monitor patients for signs and symptoms of hypotension.
Pharmacological Profile: Pharmacokinetics and Pharmacodynamics
Pharmacodynamics (PD)
The primary pharmacodynamic effect of SEPHIENCE is the dose-dependent reduction of blood phenylalanine concentrations. From a cardiac safety perspective, thorough QT studies showed that SEPHIENCE does not cause any clinically significant prolongation of the QTc interval at the maximum recommended dose.
Pharmacokinetics (PK)
- Absorption and Metabolism: Following oral administration, sepiapterin is absorbed and reaches its maximum plasma concentration (Tmax) in approximately 2 hours. It then undergoes rapid, two-step metabolism via sepiapterin reductase (SR) and dihydrofolate reductase (DHFR) to form the pharmacologically active metabolite, BH4. The systemic exposure of the parent drug is minimal, generally less than 2% of the exposure of BH4, which is the primary driver of the therapeutic effect. BH4 reaches its Tmax at approximately 4 hours.
- Impact of Food: Food significantly influences the pharmacokinetic profile of SEPHIENCE. This is a critical point for patient counseling. Administration with a high-fat, high-calorie meal can increase the total exposure (AUC) of BH4 by 251% to 284% compared to fasted conditions. This substantial increase in bioavailability is the basis for the firm recommendation to administer SEPHIENCE with food.
- Distribution: Importantly, studies have detected an increase in the active metabolite BH4 in the cerebrospinal fluid following oral administration of sepiapterin. This confirms that the therapy acts centrally, delivering the active cofactor to the brain where it is most needed to protect against the neurotoxic effects of high Phe levels.
- Elimination: The apparent terminal half-life of BH4 is approximately 5 hours in adult patients with PKU.
Regulatory Landscape and Future Outlook
Special regulatory designations often facilitate the development of therapies for rare diseases. SEPHIENCE benefited from this pathway, receiving an
‘Orphan Medicine’ designation from the European Medicines Agency (EMA) on May 20, 2021. This status encourages the development of drugs for conditions that are rare, life-threatening, or chronically debilitating.
SEPHIENCE is poised for a global launch, with key approval milestones including:
- United States (FDA): Initial U.S. Approval is set for 2025.
- European Union (EMA): Marketing Authorisation was granted on June 19, 2025.
The advent of SEPHIENCE marks a pivotal moment in the management of PKU. Its potent efficacy, novel mechanism of action, and demonstrated benefit in previously hard-to-treat patients suggest it could significantly alter the treatment paradigm. For many patients in the US, India, and around the world, this therapy may offer the potential for greater dietary freedom, a reduced treatment burden, and a better long-term prognosis.
FAQ
What is the primary mechanism that distinguishes SEPHIENCE (sepiapterin) from older therapies?
SEPHIENCE has a dual mechanism of action. Like older therapies, it acts as a precursor to the essential enzyme cofactor tetrahydrobiopterin (BH4). However, it is also believed to act as a pharmacological chaperone, directly attaching to and stabilizing the deficient phenylalanine hydroxylase (PAH) enzyme to increase its activity. This dual mechanism may explain its efficacy in patients who were previously non-responsive to other HPA medications like sapropterin.
What is the most critical administration instruction for patients taking SEPHIENCE?
The most critical instruction is to administer SEPHIENCE once daily with food. Pharmacokinetic studies show that administration with food significantly increases the bioavailability of its active metabolite, BH4. Compared to fasted conditions, a high-fat meal can increase total exposure (AUC) by 251% to 284%, making co-administration with food essential for achieving maximum therapeutic effect.
Conclusion
SEPHIENCE (sepiapterin) represents a significant and welcome addition to the therapeutic armamentarium for Phenylketonuria. As a highly effective, next-generation PAH activator, it addresses the underlying enzymatic defect with a sophisticated dual mechanism. Its robust clinical trial data showcase a profound ability to reduce blood phenylalanine levels, even in patients who have not responded to previous therapies.
For clinicians managing patients with PKU, a thorough understanding of its administration requirements—particularly the age-based dosing and the critical importance of co-administration with food—is essential for optimizing outcomes. While the safety profile is generally manageable, clinicians must remain vigilant in monitoring for key risks such as increased bleeding and hypophenylalaninemia. As SEPHIENCE becomes available to patients globally, it holds the promise of transforming lives, offering a pathway to better metabolic control and a brighter, neurologically healthier future for the PKU community.
References
- U.S. Food and Drug Administration (FDA) – Drugs@FDA Database
- European Medicines Agency (EMA) – Human Medicines
- National Organization for Rare Disorders (NORD) – Phenylketonuria Page
- National PKU Alliance (NPKUA)
- Indian Council of Medical Research (ICMR) – Newborn Screening Guidelines
Disclaimer:
Disclaimer: All the information and articles available on this site are for educational purposes only. The information given here should not be used for the diagnosis or treatment of any health problem or disease without expert advice. The advice of a qualified medical practitioner should always be sought for medical examination and treatment.
Medically Reviewed by:

Dr. Yogesh Chaudhary (B. Pharma)
Senior Pharmacist at S.N. Medical College, Agra-(UP)