On May 08, 2025 , the FDA granted accelerated approval to a novel oral therapy for a rare form of ovarian cancer. Avutometinib (a “RAF-MEK clamp” inhibitor) and Defactinib (a FAK inhibitor) are now co-packaged as Avmapki Fakzynja Co-Pack and approved for adults with KRAS-mutated recurrent low-grade serous ovarian cancer (LGSOC) who have had prior systemic treatment. This marks a first-of-its-kind novel–novel combination approval in oncology. The approval is based on the phase 2 RAMP-201 trial showing encouraging response rates in this hard-to-treat population.
LGSOC is a rare, slow-growing subtype of ovarian cancer (approx. 5–10% of all serous ovarian cancers), typically seen in younger women. Unlike high-grade ovarian cancers, LGSOC is relatively chemoresistant and often expresses hormone receptors. Importantly, many LGSOCs harbor KRAS mutations (estimated 20–40% of cases). This has spurred interest in targeted therapies against the MAPK pathway (which includes RAS/RAF/MEK/ERK).
Previously, treatments for recurrent LGSOC relied on surgery, standard chemotherapy (platinum agents), or off-label use of endocrine and targeted drugs. The MEK inhibitor trametinib showed significantly longer progression-free survival (PFS) than chemotherapy or hormone therapy in a randomized trial (median PFS 13.0 vs 7.2 months; hazard ratio 0.48), establishing MEK inhibition as a new standard in relapse. Building on this, Verastem’s combination of a novel MEK/RAF “glue” inhibitor (avutometinib) with a FAK inhibitor (defactinib) aims to further improve outcomes, especially in KRAS-mutant tumors.
This blog post explains the clinical evidence behind Avutometinib + Defactinib (Avmapki + Fakzynja), its safety profile, how it fits in the treatment landscape, and practical guidance for patients and providers. We also discuss the broader significance of targeting KRAS-mutant LGSOC.
What Is Low-Grade Serous Ovarian Cancer (LGSOC)?
LGSOC is a rare form of epithelial ovarian cancer, distinct from the more common high-grade serous subtype. It accounts for only 5–10% of serous ovarian cancers (roughly 2–5% of all ovarian cancers). Patients are often diagnosed at a younger age (mid-50s on average), and the disease tends to grow slowly. However, LGSOC is prone to recurrence and, unlike high-grade disease, it does not respond well to standard chemotherapy.
A hallmark of LGSOC is frequent activation of the MAPK pathway. Studies report that 20–40% of LGSOC tumors have activating KRAS mutations, while others may have NRAS or BRAF mutations. Hormone receptors (estrogen/progesterone) are also often expressed. Because of these molecular features, endocrine therapy (e.g., letrozole, tamoxifen) has been tried with modest success, and targeted inhibitors of MEK (a key MAPK protein) emerged as promising options.
Until recently, no FDA-approved therapy specifically targeted KRAS-mutated LGSOC. Hormone therapy may slow the disease in some patients, but response rates are generally low. Clinical trials like GOG-281/LOGS showed that trametinib (a MEK inhibitor) significantly improved outcomes: median PFS of 13.0 months versus 7.2 months for standard care. This led to trametinib’s acceptance in practice for recurrent LGSOC. The new Avutometinib + Defactinib combo builds on this by hitting the pathway in two ways, potentially overcoming resistance and improving response (see next section).
Also Read: Discover BLUJEPA Gepotidacin: A New Era in Uncomplicated UTI Treatment
Avutometinib and Defactinib: How They Work
Avutometinib (previously VS-6766) is a novel MEK/RAF pathway inhibitor. Uniquely, it is an “allosteric MEK inhibitor” that binds MEK in a complex with RAF, locking them together and preventing downstream signaling. In effect, it not only inhibits MEK but also blocks feedback activation of RAF that often occurs with other MEK inhibitors. Defactinib (formerly VS-6063) is a FAK (focal adhesion kinase) inhibitor. FAK plays a role in cell adhesion, survival, and migration; cancer cells often upregulate FAK signaling to compensate when the MAPK pathway is blocked.
The rationale for combining them emerged from preclinical and early clinical data: blocking both MEK and FAK simultaneously may be synergistic. As one commentator noted, the phase 2 data “showed that the combination was more effective” than MEK inhibition alone. This combination strategy is novel because neither drug was previously FDA-approved on its own. The FDA’s green light for this “novel–novel” combo highlights confidence in targeting KRAS-driven LGSOC through dual inhibition.
Clinical Efficacy: RAMP-201 Trial Results
The FDA’s decision was based primarily on the RAMP-201 trial (NCT04625270), an open-label, multi-center study of the combination in recurrent LGSOC. In this phase 2 trial, 57 patients with measurable KRAS-mutant LGSOC (after ≥1 prior systemic therapy) received Avutometinib 3.2 mg twice weekly (on Days 1 and 4) plus Defactinib 200 mg twice daily, in cycles of 3 weeks on/1 week off.
The primary endpoint was confirmed overall response rate (ORR) by independent review using RECIST 1.1. The key findings were impressive in this difficult-to-treat population:
- Confirmed ORR: 44% (25 of 57 patients; 95% CI 31–58%). This included 4% complete responses and 40% partial responses.
- Disease Control: An additional approximately 49% of patients had stable disease, yielding a disease control rate (CR+PR+SD) around 93%. Even among the entire study population (KRAS-mutant + wild-type, N=109), the ORR was 31%.
- Duration of Response (DOR): Very durable. The median DOR in KRAS-mutant patients was reported as 31.1 months (range 14.8–31.1). In contrast, KRAS wild-type patients had shorter DOR (~9.2 months).
- Progression-Free Survival (PFS): Median PFS was 22.0 months (95% CI 11.1–36.6) in the KRAS-mutant subgroup, versus 12.8 months in the KRAS wild-type. For all patients on the combo (KRAS-mutant + wild-type), median PFS was 12.9 months.
- Response by KRAS status: Responses were much higher in KRAS-mutated tumors. ORR was 44% (25/57) in KRAS-mutants vs 17% (9/52) in KRAS wild-type. Median DOR and PFS were also longer in the KRAS-mutant group. The FDA, therefore, approved the drug only for the KRAS-mutant setting, pending confirmatory trials.
In practical terms, nearly half of KRAS-mutant LGSOC patients responded to this oral regimen, and many responses were long-lasting. For context, in the prior standard (trametinib alone), ORR was about 44% in KRAS+ patients, but PFS was 13.0 months. The Avutometinib/Defactinib combo notably extended PFS to about 22 months in KRAS-mutants, though cross-trial comparisons should be cautious.
The efficacy data came from the primary analysis of RAMP-201 and were presented at the IGCS 2024 meeting. The FDA reviewed them under its Real-Time Oncology Review (RTOR) pilot, allowing accelerated approval based on tumor responses. A confirmatory phase 3 trial (RAMP-301) is planned to verify these benefits and possibly expand use regardless of KRAS status.
Comparison to Other Therapies
Before Avutometinib/Defactinib, options for recurrent LGSOC were limited. Platinum chemo often has modest effect, and many patients are treated with hormonal therapy. For example, letrozole or tamoxifen can sometimes stabilize disease, but true response rates are low (single digits) and evidence is from small studies.
The MEK inhibitor trametinib has been a game-changer. In the GOG-281/LOGS trial, trametinib significantly beat standard treatments (chemo/hormones) in recurrent LGSOC: median PFS 13.0 vs 7.2 months, and the FDA now recognizes trametinib for LGSOC. The ORR with trametinib was about 44% (similar to Avu/Def) in unselected patients, though detailed breakdowns by KRAS status were not a primary focus.
How does Avutometinib+Defactinib stack up?
The combination has a comparable ORR (44% in KRAS-mutant LGSOC) but appears to deliver longer progression-free intervals in this genotype-selected group (median PFS approximately 22 months). Importantly, the Avu/Def regimen is given intermittently (Day 1 and 4 of each week for 3 weeks) rather than daily, potentially reducing continuous MEK inhibition toxicities. It remains to be seen in head-to-head studies whether dual MAPK+FAK blockade is superior to single-agent MEK inhibition or maintenance hormone therapy.
Another point of comparison is side-effect burden (see Safety section below) – MEK inhibitors often cause rash, diarrhea, and edema. The Avu/Def combo shows a similar spectrum, though with its own twist (e.g. high rates of creatine kinase elevation). In practice, the choice may come down to a patient’s mutation status, prior therapies, and tolerance of MEK-inhibitor class effects.
Overall, Avutometinib+Defactinib fills an unmet need. There are currently no FDA-approved treatments specifically for KRAS-mutant LGSOC, so oncologists might use this new combo in eligible patients. Its approval reinforces the paradigm of precision therapy – targeting a key mutation (KRAS/MAPK) in a cancer subtype that lacks effective standard options.
Efficacy Results Summary
| Population | ORR (confirmed) | Median PFS | Median DOR |
|---|---|---|---|
| KRAS-mutant LGSOC (N=57) | 44% (25/57) | 22.0 months | 31.1 months |
| All LGSOC (KRAS-mutant + wild-type, N=109) | 31% (34/109) | 12.9 months | 31.1 months |
| KRAS–wild-type LGSOC (N=52) | 17% (9/52) | 12.8 months | 9.2 months |
Table: Key efficacy outcomes from the phase 2 RAMP-201 trial. ORR = overall response rate; PFS = progression-free survival; DOR = duration of response.
Safety and Side Effects
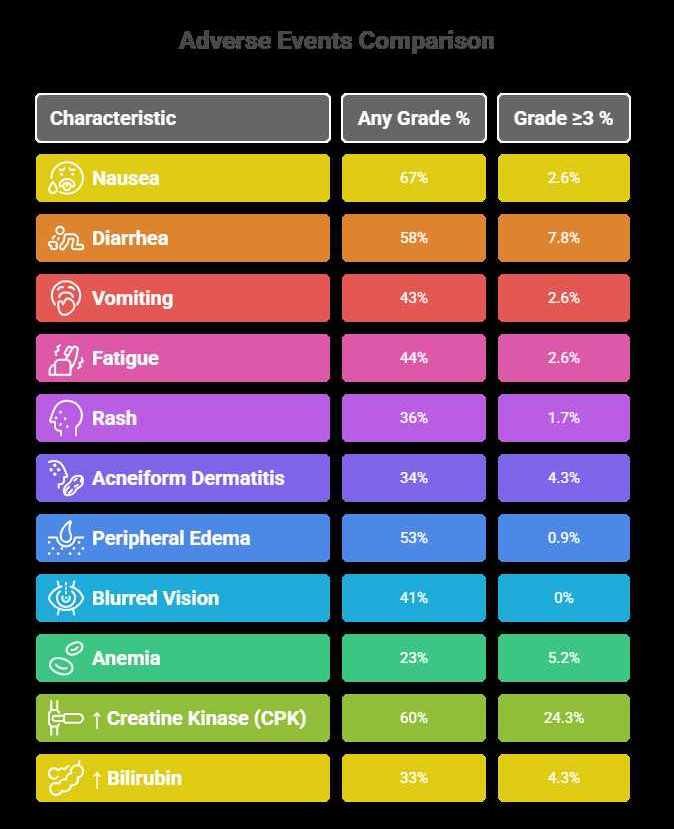
The safety profile of Avutometinib + Defactinib is generally consistent with the known effects of MEK and FAK inhibition, with no unexpected signals in trials. In RAMP-201, serious adverse events occurred in about 32% of patients, with serious reactions (any cause) including sepsis, intestinal obstruction, and pyelonephritis (each ~3–9%). Treatment discontinuation due to AEs was approximately 10%.
The most common side effects (all grades) seen (≥25% of patients) were:
- Gastrointestinal: Nausea (67%), diarrhea (58%), vomiting (43%), abdominal pain, dyspepsia.
- Skin/Mucosa: Rash (35%), acneiform dermatitis (34%), dry skin (26%), pruritus.
- Fatigue & Edema: Fatigue (44%), peripheral edema (53%).
- Muscle/Metabolic: Elevation of creatine kinase (60%), which can indicate muscle soreness.
- Hematology/Lab: Decreased hemoglobin (anemia, 23%), increased AST/ALT, increased bilirubin, increased triglycerides, lymphopenia, thrombocytopenia, neutropenia.
- Vision: Blurred vision (41%) and other ocular issues were noted; prompt eye exams are recommended (see Counseling).
Grade ≥3 (severe) events were less common: for example, grade 3-4 nausea was only 2.6%, diarrhea 7.8%, and grade 3 CPK elevations 24%. Serious treatment-related AEs were reported in about 7% of patients (abdominal pain being one occurring >1 patient). Importantly, no new safety signals beyond known MEK/FAK inhibitor effects emerged in the combo.
In summary, patients should be counseled on nausea, diarrhea, rash, fatigue, and muscle aches as the most likely side effects. Supportive care (anti-nausea meds, anti-diarrheal, skin creams) is essential. The prescribing information specifically recommends prophylactic skin therapy: RAMP-201 patients used topical corticosteroids (on face, scalp, trunk) and oral antibiotics from treatment start to prevent rash. Patients should also be advised to limit sun exposure and use SPF 30+ sunscreen. Liver enzymes and blood counts should be monitored regularly (as AST/ALT elevations and cytopenias can occur).
Finally, embryo-fetal toxicity is a critical risk: the drug can harm a fetus (Adverse Reaction: embryofetal toxicity). Both men and women should use effective contraception – women during and for 1 month after treatment, men during and for 4 months after last dose.
Dosage, Administration & Patient Guidance
Avmapki Fakzynja Co-Pack is supplied as two separate products (avutometinib capsules and defactinib tablets) to be taken together on the same schedule. The recommended regimen is:
- Avutometinib: 3.2 mg (four 0.8 mg capsules) twice weekly, on Day 1 and Day 4 of each week for the first 3 weeks of a 4-week cycle.
- Defactinib: 200 mg (one tablet) twice daily for the first 3 weeks of each 4-week cycle.
- Then follow 1 week off treatment (i.e., cycle = 3 weeks on drug, 1 week off) before repeating. Continue until disease progression or intolerable toxicity.
This intermittent schedule (3-weeks-on/1-week-off) helps manage toxicity while maintaining efficacy. Patients should be given specific calendars to track dosing days. Avutometinib capsules can be taken with or without food, but defactinib tablets have no special requirement either. If a dose is missed on the scheduled day, instructions allow it to be taken as soon as possible that day, but missed doses should generally not be made up beyond that. (Refer to full prescribing information for detailed dose-modification guidelines in case of side effects.)
Patient tips:
Swallow capsules/tablets whole with water. Maintain the 4-week-on/off cycle rhythm. Keep track of eye exams and skin care regimen. Avoid grapefruit juice and St. John’s Wort (which could affect metabolism), although no strong interactions are noted on the label.
Patient Counseling Points
When starting Avutometinib + Defactinib, healthcare providers should counsel patients on the following key points:
- Side Effects & Supportive Care: Emphasize management of the common AEs (nausea, diarrhea, rash, fatigue, muscle aches). Prescribe anti-nausea (e.g., ondansetron) and anti-diarrheal (e.g., loperamide) medications as needed. For rash or acneiform dermatitis, recommend non-irritating moisturizers and prophylactic steroid creams (topical methylprednisolone) on the face and upper body. Inform patients about the high chance of muscle pain/CPK elevation and to report any unexplained muscle weakness or dark urine. Schedule regular laboratory tests (CK, liver enzymes, blood counts).
- Skin & Sun Protection: Counsel patients to use sunscreen (SPF ≥30) daily and minimize sun exposure, as skin sensitivity is increased.
- Eye Safety: The trial noted some visual disturbances. Patients should get an eye exam before and periodically during treatment, and report any vision changes immediately (blurriness, eye pain, floaters).
- Contraception: Because of fetal risk, women of childbearing potential must use effective contraception during therapy and for 1 month after the last dose. Men should use condoms and not father a child during treatment and for 4 months after the last dose.
- Drug Interactions: Advise patients to discuss all medications and supplements. For example, anticoagulant use (warfarin) was a trial exclusion, so check if defactinib affects clotting.
- Adherence: Stress the importance of sticking to the odd dosing schedule (e.g., taking Avutometinib only on two days a week). Provide a written schedule or app reminder. The defactinib twice-daily schedule on active weeks should be followed rigorously.
- When to Call the Doctor: If symptoms like severe rash, persistent diarrhea, or muscle pain occur, adjust medication only under medical guidance (some toxicities may require dose reduction rather than stopping). Any sign of infection or fever should prompt immediate contact, given the risk of sepsis in a small fraction of patients.
A pamphlet or summary sheet can reinforce these counseling points. Involvement of a specialty pharmacist or nurse for education can be very helpful. Because this is an oral regimen, patients will manage it at home, so clear guidance on what to expect (and when to reach out) is critical.
Market Potential and Significance
Although LGSOC is rare, the market potential is meaningful for this specific indication. Ovarian cancer affects approx 20,000 women annually in the U.S., so LGSOC (approx 5–10% of serous cases) might represent approx 1,000–2,000 new patients per year. Among these, roughly one-third have KRAS mutations, the eligible population for Avmapki Fakzynja. In Europe and Asia, similar numbers apply. Worldwide, total patient-years could be in the thousands. Given the lack of tailored options for KRAS-mutant LGSOC, nearly all eligible patients may be considered for this targeted therapy once it is available.
From a drug development perspective, the accelerated approval means Verastem (the company) can capture this niche market relatively quickly, particularly as there are currently no FDA-approved drugs specifically for KRAS-mutant LGSOC. If phase 3 confirmatory trials (RAMP-301) succeed, the indication could be solidified and possibly expanded to any LGSOC patient, not just KRAS-mutant. The pricing will likely reflect its status as a precision therapy for an orphan cancer population.
For oncologists and the pharmaceutical industry, this approval is noteworthy: it validates the strategy of “double targeting” a pathway in a mutation-defined subgroup. It may encourage more trials of similar novel-novel combinations in other cancers. The FDA’s support of this regimen signals a willingness to approve co-developed combos, which historically is challenging.
In the broader oncology field, success with Avutometinib + Defactinib could invigorate research on KRAS-driven tumors (beyond LGSOC), as inhibiting downstream nodes of the KRAS pathway and parallel survival pathways (like FAK) could be applied in lung, colorectal, pancreatic, or other KRAS-mutant cancers. Indeed, Avutometinib itself has been studied in RAS-mutant lung cancer.
Finally, for patients and advocacy groups, this provides hope and momentum. As one investor newsletter put it: “We believe [the combo] has the potential to change the treatment paradigm for recurrent KRAS-mutant LGSOC”. With this approval, patients who previously had no targeted choices for their specific tumor type now have an FDA-sanctioned option.
Safety Profile Table
| Adverse Event | Any Grade % | Grade ≥3 % |
|---|---|---|
| Nausea | 67% | 2.6% |
| Diarrhea | 58% | 7.8% |
| Vomiting | 43% | 2.6% |
| Fatigue | 44% | 2.6% |
| Rash | 36% | 1.7% |
| Acneiform Dermatitis | 34% | 4.3% |
| Peripheral Edema | 53% | 0.9% |
| Blurred Vision | 41% | 0% |
| Anemia | 23% | 5.2% |
| ↑ Creatine Kinase (CPK) | 60% | 24.3% |
| ↑ Bilirubin | 33% | 4.3% |
| ↑ AST/ALT (Liver Enzymes) | 31% (AST) | 1.7% (AST) |
Conclusion
The approval of Avutometinib + Defactinib (Avmapki Fakzynja Co-Pack) offers a promising new targeted option for a rare, genomically-defined subset of ovarian cancer patients. By building on the biology of KRAS-driven LGSOC, this combination achieved durable responses in a heavily pretreated population. While managing its side effects requires vigilance (especially rash and GI symptoms), the overall safety appears manageable with supportive care.
For oncologists and patients, this represents a shift towards personalized therapy: patients with KRAS-mutant LGSOC now have an evidence-backed, FDA-approved regimen tailored to their tumor’s biology. Ongoing trials and real-world experience will clarify how this combo compares head-to-head with existing options and whether it can benefit a broader group of LGSOC patients.
In the broader oncology landscape, the success of this “MEK inhibitor + FAK inhibitor” strategy may pave the way for novel combination approaches in other KRAS-mutant cancers. As the first-of-its-kind novel–novel drug approval, it also sets a precedent that tackling compensatory survival pathways can unlock potent new regimens.
In summary, Avutometinib/Defactinib brings new hope to patients with KRAS+ LGSOC. With careful monitoring and patient education, the benefits of this therapy can be maximized. Stakeholders across oncology—from clinicians to researchers—will be watching closely as this innovative treatment reaches practice and potentially changes the standard of care for a difficult-to-treat cancer subtype.
References
- Advances in precision therapy of low-grade serous ovarian cancer: A review-[https://journals.lww.com/]
- Current concept of low-grade serous ovarian carcinoma-[https://tcr.amegroups.org/]
- Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): an international, randomised, open-label, multicentre, phase 2/3 trial-[https://pubmed.ncbi.nlm.nih.gov/35123694/]
- What to know about low-grade serous ovarian cancer-[https://www.medicalnewstoday.com/articles/low-grade-serous-ovarian-cancer]
- FDA grants accelerated approval to the combination of avutometinib and defactinib for KRAS-mutated recurrent low-grade serous ovarian cancer-[https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-combination-avutometinib-and-defactinib-kras-mutated-recurrent-low]
- Avutometinib Combo Yields Responses in Low-Grade Serous Ovarian Cancer-[https://www.cancernetwork.com/view/avutometinib-combo-yields-responses-in-low-grade-serous-ovarian-cancer]