Schedule-G drugs represent a crucial category within the regulatory framework of the Indian pharmaceutical industry, established under the Drugs and Cosmetics Rules, 1945. This schedule is designed to classify medications that, while essential for treating serious and chronic conditions, possess inherent risks, complex dosing requirements, or the potential for severe adverse effects, making strict professional oversight mandatory.
The core purpose of the Schedule-G classification is to ensure the rational and safe use of these high-potency agents. Historically, this classification was introduced to govern drugs that necessitated administration under a medical practitioner’s guidance, preventing self-medication that could lead to toxicity, irreversible harm, or treatment failure. Unlike drugs of abuse, the emphasis of Schedule-G is on inherent therapeutic risk requiring expert management.
Under the Drugs and Cosmetics Rules, 1945, Schedule-G enforces a mandatory and conspicuous labeling requirement that acts as the primary safety barrier, alerting both pharmacists and consumers that the preparation is dangerous without expert oversight.

The Definitive Schedule-G Drugs List and Therapeutic Categories
The list of Schedule-G drugs, while occasionally updated by the Central Drugs Standard Control Organization (CDSCO) and the Ministry of Health, generally comprises potent agents used for managing non-communicable diseases (NCDs) and life-threatening illnesses. When official government lists are fragmented, comprehensive pharmaceutical portals confirm the inclusion of drugs based on their mechanism of action and regulatory history.
These drugs bear a special warning on the outer label of their packing as “Caution: It is dangerous to take this preparation except under medical supervision”.
The drugs in this Schedule fall into distinct therapeutic categories, highlighting the specialized nature of their use:
| Therapeutic Category | Example Drug Names (Key Active Ingredients) | Rationale for Inclusion |
| Anticancer Agents (Chemotherapy) | Aminopterin, L-Asparaginase, Bleomycin, Busulphan, Cis-Platin, Chlorambucil, Doxorubicin, Mercaptopurine, Hydroxyurea, Mustine. | Severe toxicity (nephrotoxicity, cardiotoxicity, myelosuppression), narrow therapeutic index, requires specialized monitoring. |
| Antidiabetics & Hormonal Agents | Insulin (all types), Metformin Hydrochloride, Glibenclamide, Chlorpropamide, Disodium Stilboestrol Diphosphate. | Risk of severe hypoglycemia (insulin/sulfonylureas) or lactic acidosis (Metformin), complex dosing, vital for chronic disease management. |
| Central Nervous System (CNS) Agents | Ethosuximide, Hydantoin (Derivatives), Methsuximide, Phenacemide, Primidone, Troxidone. | Potential for severe blood dyscrasias, neurotoxicity, psychiatric side effects, and complex management of seizures. |
| Cardiovascular/Diuretics | Chlorothiazide, Chlorthalidone, Ethacrynic Acid, Quinthazone. | Risk of profound fluid loss, severe electrolyte imbalance (hypokalemia), and organ toxicity (ototoxicity for Ethacrynic Acid). |
| Antihistamines | Antazoline, Bromodiphenhydramine, Chlorpheniramine, Diphenhydramine. | Included primarily for their potent sedative/anticholinergic effects, necessitating supervision to prevent accidents or misuse. |

Schedule-G Drugs List(as per D&C Rules, 1945)
This list comprises highly potent substances where unsupervised use carries significant inherent risk of harm or requires specialized medical management.
I. Antineoplastic (Chemotherapy) and Related Agents
| Drug Substance | Therapeutic Use Rationale |
| Aminopterin | Folic acid antagonist, immunosuppressant, high toxicity. |
| L-Asparaginase | Enzyme used in leukemia treatment; high risk of hypersensitivity. |
| Bleomycin | Antitumor antibiotic; risk of pulmonary fibrosis. |
| Busulphan; its salts | Alkylating agent; severe myelosuppression and organ toxicity. |
| Chlorambucil; its salts | Alkylating agent; severe myelosuppression. |
| Cis-Platin | Chemotherapy agent; severe nephrotoxicity and neurotoxicity. |
| Cyclophosphamide; its salts | Alkylating agent; hemorrhagic cystitis risk. |
| Cytarabine | Pyrimidine analog, used for leukemias; myelosuppression. |
| Daunorubicin | Anthracycline antibiotic; dose-dependent cardiotoxicity. |
| Doxorubicin Hydrochloride | Anthracycline antibiotic; dose-dependent cardiotoxicity. |
| Hydroxyurea | Antineoplastic agent; severe myelosuppression. |
| Lomustine Hydrochloride | Alkylating agent; delayed and cumulative myelosuppression. |
| Mannomustine; its salts | Nitrogen mustard derivative; severe toxicity. |
| Mercaptopurine; its salts | Purine analog; severe myelosuppression and hepatotoxicity. |
| Mustine; its salts | Nitrogen mustard; highly toxic, vesicant. |
| Procarbazine Hydrochloride | MAOI activity and myelosuppression. |
| Sarcolysine | Alkylating agent; neurotoxicity and myelosuppression. |
| Tamoxifen Citrate | Anti-estrogen; hormonal therapy for breast cancer, high risk profile. |
| Testolactone | Aromatase inhibitor; hormonal agent. |
| Thiotepa | Alkylating agent; severe myelosuppression. |
| Tretamine; its salts | Alkylating agent; severe myelosuppression. |
| Sodium-2-Mercaptoethanesulfonate (Mesna) | Uroprotectant (used with cyclophosphamide/ifosfamide). |
II. Antidiabetic (Hypoglycemic) and Hormonal Agents
| Drug Substance | Therapeutic Use Rationale |
| Insulin, all types | High risk of severe, life-threatening hypoglycemia. |
| Metformin; its salts | Biguanide; risk of lactic acidosis (especially in renal impairment). |
| Glibenclamide | Sulfonylurea; high risk of prolonged hypoglycemia. |
| Carbutamide | Sulfonylurea (historical use); risk of hypoglycemia. |
| Chlorpropamide; its salts | Sulfonylurea; high risk of prolonged hypoglycemia. |
| Phenformin; its salts | Biguanide (largely withdrawn); high risk of lactic acidosis. |
| Tolbutamide | Sulfonylurea; risk of hypoglycemia. |
| Disodium Stilboestrol Diphosphate | Estrogen derivative; hormonal agent with cardiovascular risk. |
III. Central Nervous System (CNS) Agents
| Drug Substance | Therapeutic Use Rationale |
| Ethosuximide | Anticonvulsant for absence seizures; risk of blood dyscrasias. |
| Hydantoin; its salts; its derivatives, their salts | Anticonvulsant class; complex drug interactions and narrow index. |
| Methsuximide | Anticonvulsant; risk of blood dyscrasias and behavioral changes. |
| Paramethadione | Anticonvulsant; teratogenic and high toxicity risks. |
| Phenacemide | Anticonvulsant (last resort); high risk of psychiatric/liver toxicity. |
| 5-Phenylhydantoin; its alkyl and aryl derivatives; its salts | Anticonvulsant class. |
| Primadone | Anticonvulsant; significant sedation and potential blood dyscrasias. |
| Troxidone | Anticonvulsant (historical); high risk of severe toxicity. |
| Di-Isopropyl Eluorophosphate | Potent cholinesterase inhibitor (used in glaucoma). |
IV. Cardiovascular Agents and Diuretics
| Drug Substance | Therapeutic Use Rationale |
| Chlorothiazide and other derivatives of 1, 2, 4 benzothiadiazine | Diuretic; risk of severe electrolyte imbalance. |
| Chlorthalidone and other derivatives of Chlorobenzene compound | Diuretic; risk of severe electrolyte imbalance. |
| Ethacrynic Acid; its salts | Potent loop diuretic; risk of ototoxicity and profound fluid loss. |
| Quinthazone | Diuretic; risk of electrolyte imbalance. |
V. Antihistaminic Substances
The schedule includes the following antihistamine substances, their salts, their derivatives, and the salts of their derivatives:
- Antazoline
- Bromodiphenhydramine
- Buclizine
- Chlorcyclizine
- Chlorpheniramine
- Clemizole
- Cyproheptadine
- Diphenhydramine
- Diphenylpyraline
- Doxylamine Succinate
- Isothipendyl
- Mebhydrolin Napadisylate
- Meclozine
- Phenindamine
- Pheniramine
- Promethazine
- Thenalidine
- Triprolidine
- Substances being tetra-N-Subs. derivatives of Ethylene Diamine or Prophylenediamine.
Recent Regulatory Context
A significant recent focus (as seen in proposals around July 2024/2025) has been on controlling the promotion of these critical drugs. The Ministry of Health and Family Welfare is actively considering amending the Drugs and Cosmetics Rules, 1945, to prohibit advertisements of Schedule-G drugs (such as Metformin and Insulin) without prior approval from the Central Licencing Authority. This move, supported by the Drugs Technical Advisory Board (DTAB), seeks to prevent misleading claims that could encourage self-medication, underscoring the government’s continued commitment to ensuring medical supervision for this category.
Example of Schedule G drug warning:

Required Warning Labels for Schedule-G Drugs
The labeling requirement for Schedule-G drugs is prescribed under Rule 97 of the Drugs and Cosmetics Rules, 1945. This rule mandates a specific and unambiguous cautionary statement to be displayed prominently on the container and the packaging (label).
Exact Language Required by Law
The law requires the following exact statement to be printed on the label:
“Caution: It is dangerous to take this preparation except under medical supervision”

Visual Label Specifications
While Schedule G does not enforce the universal “red vertical line” or red border required for some other sensitive schedules, Rule 97 specifies the placement and presentation of the caution:
- Conspicuous Placement: The warning must be printed in a manner that makes it clearly visible and easily legible.
- Surrounded by a Line: The caution text must be surrounded by a line within which there shall be no other words. This creates a designated, separate box for the warning, ensuring it is isolated from other label text.
This strict visual isolation ensures that the message of danger and the necessity of medical supervision cannot be missed by the dispenser or the patient.
Enforcement and Mislabeling Controversies
Regulatory scrutiny often focuses on whether manufacturers fully comply with the Rule 97 mandate, especially in small-volume packaging. Studies assessing label compliance in India have noted that while the text compliance for Schedule G drugs is often 100%, minor non-compliance often occurs with other essential label particulars.
Controversies over Schedule G often revolve around the recent push to control their advertising. The draft notification to restrict ads highlights the government’s concern that even truthful claims, if widely advertised to the public, can lead consumers to inappropriately request or attempt to self-medicate with high-risk drugs like Metformin or potent antihistamines, thereby undermining the supervisory intent of the schedule.
Specific Medical Uses and Risks of Schedule-G Drugs
Schedule-G drugs require medical supervision because their mechanism of action or therapeutic window necessitates careful monitoring to balance efficacy against severe, dose-dependent side effects.
Why Supervision is Essential
- Narrow Therapeutic Index: Many Schedule-G drugs, especially chemotherapeutic and anticonvulsant agents, have a narrow margin between a therapeutic dose and a toxic dose. Slight variations can lead to severe harm.
- Severe Organ Toxicity: Drugs like Doxorubicin (Chemotherapy) can cause irreversible cardiotoxicity (heart damage), while Cisplatin can cause severe nephrotoxicity (kidney damage). These require pre-treatment assessment and continuous monitoring of organ function.
- Complex Dosing and Drug Interactions: Agents like Insulin require highly individualized, dynamic dosing based on daily blood glucose levels, diet, and activity. Drugs like Metformin carry the rare but fatal risk of lactic acidosis, which is significantly increased when the patient has impaired renal function, requiring regular checks.
Clinical Scenarios Illustrating Importance
- Scenario 1: Cancer Chemotherapy (Cis-Platin): A patient is prescribed Cis-Platin for testicular cancer. Without continuous medical supervision, including frequent blood tests and hydration protocols, the patient could suffer acute kidney injury. The drug’s inherent toxicity means that while it is essential to destroy cancer cells, the supervision ensures the dose is adjusted immediately if renal markers (creatinine, BUN) rise dangerously, saving the patient’s life.
- Scenario 2: Antidiabetic Management (Insulin): An elderly patient begins insulin therapy. Without medical supervision and proper education, the patient might skip a meal but take the full dose, leading to severe, life-threatening hypoglycemia (dangerously low blood sugar) requiring emergency hospitalization. The supervision ensures proper titration, injection technique, and patient education.
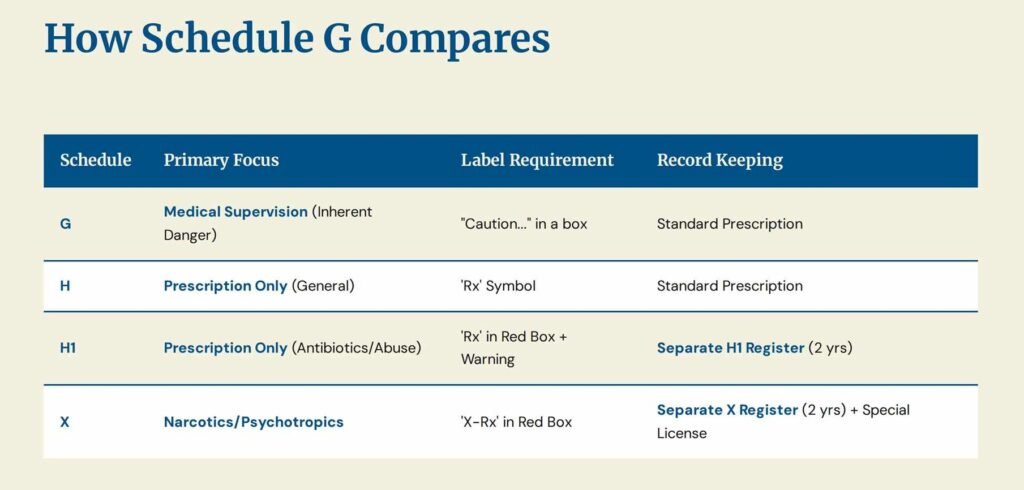
Distinguishing Schedule-G from Schedules H, H1, and X
The Indian regulatory framework classifies drugs based on the level of restriction required for their distribution and sale. Schedule-G is distinct from the more restrictive schedules:
| Schedule | Primary Regulatory Focus | Key Warning/Symbol | Prescription/Sale Restriction |
| Schedule G | Mandatory Medical Supervision (Inherent Risk) | “CAUTION: It is dangerous to take this preparation except under medical supervision” | Sale restricted to prescription, but the focus is the inherent danger to the patient. |
| Schedule H | Prescription Drug (General) | Red ‘Rx’ symbol on the top left. | Must be sold only on the prescription of a Registered Medical Practitioner (RMP). |
| Schedule H1 | Prescription Drug (Stricter Tracking) | Red ‘Rx’ symbol and the specific warning in a red box. | Requires prescription. Pharmacist must maintain a separate sales register for two years and mark the prescription with a sequential number/date. (For third-gen antibiotics, certain habit-forming drugs). |
| Schedule X | Psychotropics/Narcotics (Highest Risk) | Red ‘X-Rx’ symbol. | Requires a special licence for possession/storage. Must be kept under lock and key. Retailer must preserve a copy of the prescription for two years. |
Key Difference: Schedule G focuses on inherent toxicity (e.g., chemotherapeutic toxicity), while Schedules H, H1, and X focus heavily on potential for misuse, abuse, or antimicrobial resistance.
Regulatory, Distribution, and Sales Restrictions

Prescribing and Dispensing Authority
- Prescribing: Only a Registered Medical Practitioner (RMP) can prescribe Schedule G drugs.
- Dispensing: A licensed pharmacist can dispense a Schedule G drug only against a valid RMP’s prescription. The critical dispensing responsibility is to ensure the patient is aware of the mandatory warning on the label and understands the need for medical follow-up.
Record-Keeping and Checks
Unlike Schedule H1 and X, the Drugs and Cosmetics Rules, 1945, do not explicitly mandate a specific, separate ledger for recording every sale of Schedule-G drugs by retail. However, good pharmacy practice and general licensing conditions (Rule 67) dictate that all prescription drugs must be traceable.
- Pharmacy Record-Keeping: Pharmacies must maintain standard records for all dispensed prescription drugs, including the RMP’s details, patient information, and the quantity supplied.
- Purchase Orders: Manufacturers and distributors must maintain detailed records of sales to licensed dealers or pharmacies.
Penalties for Non-Compliance
Violation of the provisions concerning Schedule G drugs, such as supplying them without a prescription or failing to affix the mandatory warning label (making it a ‘misbranded’ drug under Section 17), can lead to severe legal consequences under the Drugs and Cosmetics Act, 1940:
- Misbranding/Unlawful Sale (Section 27): Punishable with imprisonment, which may extend from three years up to five years, and a fine.
- Manufacturing/Selling spurious or adulterated drugs (Section 27): Carries even more stringent penalties, including imprisonment of up to ten years or life imprisonment in cases where the drug causes grievous bodily harm or death.
Patient Safety, Awareness, and Best Practices
Patient awareness is the final layer of defense against misuse of Schedule G drugs.
Guidance to Patients/Caregivers
- Recognize the Label: Always look for the clear warning box with the text, “Caution: It is dangerous to take this preparation except under medical supervision.”
- Strictly Follow Advice: These are not ‘as-needed’ drugs. Follow the prescribed dosage, timing, and duration exactly. Never adjust the dose or stop the medication (especially hormonal agents like Insulin or epilepsy drugs) without consulting the RMP.
- Understand Monitoring: Expect your doctor to request regular blood tests or check-ups (e.g., kidney/liver function tests for chemotherapy or HbA1c/glucose monitoring for diabetics). This monitoring is part of the treatment.
Red Flags for Consumers
Consumers should treat the following as immediate red flags:
- A pharmacist selling a Schedule G drug without a prescription.
- The mandatory cautionary warning label being obscured, missing, or printed inconspicuously.
- Any promotional material or advertisement online or in print claiming the drug can be used safely without consulting a doctor.
Comparison with International Approaches
While the exact “Schedule G” terminology is unique to India, similar supervised drug categories exist globally to manage high-risk medications:
- United States (US): The concept aligns with Legend Drugs (requiring “Rx only” or “Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian,” or similar language for human use), but is closer to the special controls placed on high-risk medications like chemotherapy (often covered by specific safety protocols like REMS—Risk Evaluation and Mitigation Strategies).
- United Kingdom (UK): The category is similar to Prescription Only Medicines (POM). Drugs that require close monitoring, like some hormonal therapies or chemotherapy agents, are governed by hospital protocols and specific dispensing rules that ensure patient supervision is maintained, much like the supervisory intent of Schedule G.
FAQ
What are the schedule g drugs side effects?
Schedule G drugs are prescribed medicines in India. They should not consume without the recommendations of a doctor. If they are used without symptoms, they can provide some severe side effects to the patient. These medicines come with a warning on the label they are packed in.
How many drugs are covered in Schedule G in India?
There are more than 60 medicines and their derivatives are covered in the Schedule G Drugs list in India.
If a drug like Insulin or Metformin is classified under Schedule G, why is it sometimes referred to as a “life-saving drug”?
The classification of a drug in Schedule G is a testament to its potency and necessity, not its dangerous nature alone. These are often essential, life-saving drugs (e.g., chemotherapy, insulin for Type 1 diabetes) required for critical illness management. They are classified in Schedule G precisely because they are so potent: they can save a life when used correctly under supervision, but they can easily cause severe harm or death (via cardiotoxicity, nephrotoxicity, or hypoglycemia) if the supervision is absent or inadequate. The Schedule ensures that their power is managed by an RMP.
References:
- Government of India (CDSCO & MoHFW). The Drugs and Cosmetics Act, 1940 and The Drugs and Cosmetics Rules, 1945 (including Rule 97 and Schedule G). Official Regulatory Text.
- Central Drugs Standard Control Organization (CDSCO) / Ministry of Health and Family Welfare. Relevant official notifications and circulars regarding drug schedules and labeling.
- Indian Pharmacopoeia Commission (IPC). General standards and classifications (supporting the Indian Pharmacopoeia).
- Scribd / SlideShare (Academic Materials). Pharmaceutical Jurisprudence presentations and documents from educational and professional sources providing the historical and detailed list of Schedule G substances.
- PubMed / PMC (National Center for Biotechnology Information). Scientific and clinical references concerning prescription drug labeling, medication errors, and the toxicology/pharmacology of Schedule G substances (e.g., Narrow Therapeutic Index agents).
- Drugs Control Media Services (DrugsControl.org). Regulatory intelligence and news on drug labeling compliance and enforcement in India.


![Schedule X Drugs List [2025 Update] - Uses & Side Effects schedule x drugs list](https://laafon.com/wp-content/uploads/2024/11/1-768x402.jpg)

