On December 30, 2025, the U.S. Food and Drug Administration (FDA) approved Nereus (tradipitant), an oral neurokinin-1 (NK-1) receptor antagonist, for the prevention of vomiting induced by motion in adults[1]. This regulatory milestone marks the first novel pharmacologic intervention for motion sickness to reach the market in over four decades, addressing a therapeutic void that has persisted since the late 1970s [2].
Developed by Vanda Pharmaceuticals, tradipitant offers a mechanism-driven approach to emesis prevention, moving beyond the systemic side effects associated with heritage antihistamines and muscarinic antagonists[3]. For healthcare professionals (HCPs) and industry stakeholders, Nereus represents a significant advancement in neuropharmacology and operational readiness for travelers in high-provocation environments[4]
Also Read: Rhapsido FDA Approval 2025: Comprehensive Analysis for Pharmaceutical Professionals
Mechanism of Action: Targeted Tachykinin Modulation
Nereus operates through a sophisticated mechanism of action that targets the sensory-motor conflict at the root of motion sickness[5].
The Role of Substance P and NK-1 Receptors
Tradipitant is a selective, high-affinity antagonist of human substance P/neurokinin-1 (NK-1) receptors[6]. Substance P is a primary neuropeptide involved in the emetic reflex; during motion provocation, sensory conflict triggers its release, which then binds to NK-1 receptors in the central nervous system (CNS) emetic centers to induce vomiting[7].
Molecular Selectivity
Unlike older therapies, tradipitant demonstrates high selectivity for the NK-1 receptor[8]. Clinical data indicates:
- Target Specificity: Tradipitant does not have affinity for NK2, NK3, serotonin (5-HT3), dopamine (D2), cholinergic, or histamine (H1) receptors[9].
- Receptor Occupancy: PET imaging studies demonstrated a dose-dependent increase in frontal cortex NK-1 receptor occupancy, reaching 93% after multiple administrations of 100 mg[10].
- CNS Penetration: Its molecular architecture, featuring multiple fluorinated aromatic rings, optimizes its ability to traverse the blood-brain barrier and reach emetic centers[11].
Clinical Trial Evidence: The Motion Series
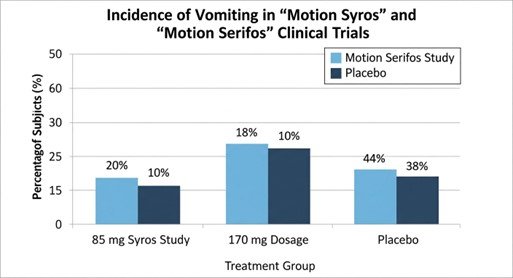
The FDA approval was supported by robust data from a clinical development program consisting of three pivotal trials, most notably the real-world provocation studies: Motion Syros and Motion Serifos[12].
Study 1: Motion Syros (NCT04327661)
This randomized, double-blind, placebo-controlled trial evaluated N=365 adult subjects with a history of motion sickness during boat trips lasting 2 to 5 hours[13].
- Primary Endpoint: Percentage of subjects with vomiting[14].
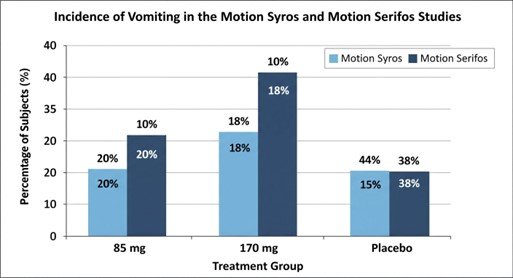
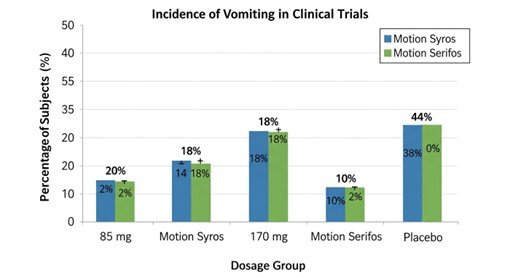
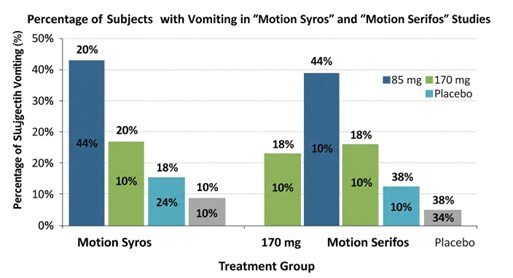
- Results (85 mg): 20% experienced vomiting vs. 44% for placebo (95% CI: -36%, -14%; p < 0.0001)[15].
- Results (170 mg): 18% experienced vomiting vs. 44% for placebo (95% CI: -37%, -15%; p < 0.0001)[16].
Study 2: Motion Serifos (NCT05903924)
Study 2 involved N=316 subjects in similar maritime conditions[17].
- Results (85 mg): 18% experienced vomiting vs. 38% for placebo (95% CI: -31%, -8%)[18].
- Results (170 mg): 10% experienced vomiting vs. 38% for placebo (95% CI: -38%, -16%; p \leq 0.0014)[19]
These trials demonstrated risk reductions of over 50–70% in vomiting incidence compared to placebo[20].
Therapeutic Landscape: Comparative Analysis
To understand the clinical impact of Nereus, it must be compared against the existing standard of care, primarily consisting of anticholinergics and first-generation antihistamines.
| Feature | Nereus (Tradipitant) | Anticholinergics (e.g., Scopolamine) | Clinical Implication |
| Pharmacologic Class | NK-1 Receptor Antagonist | Muscarinic Antagonist | Targeted vs. Systemic action. |
| Primary AE Profile | Somnolence, Fatigue, Headache | Dry mouth, blurred vision, confusion | Nereus avoids traditional “heritage” side effects. |
| Dosing Frequency | Episodic (Single dose) | Continuous (72-hour patch) or Multi-dose | Nereus is optimized for acute travel events. |
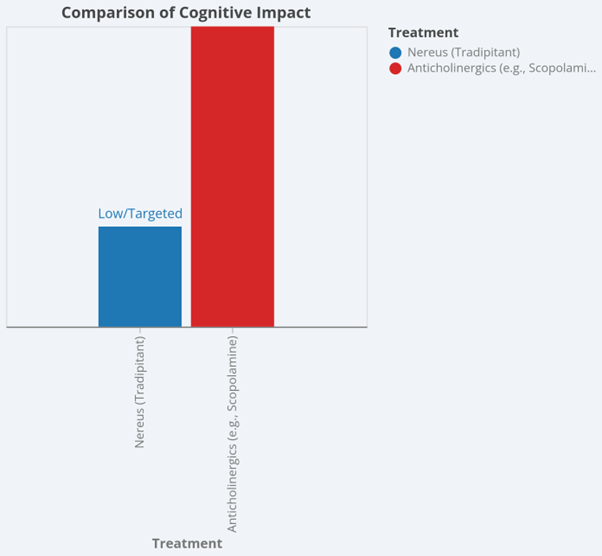
| Cognitive Impact | Low/Targeted | High (Cognitive clouding) | Essential for military and professional operators |
Nereus is positioned as a first-line prescription therapy for adults who require effective vomiting prevention without the cognitive impairment and anticholinergic burden of older agents.
Safety and Tolerability Profile
The safety of a single 85 mg or 170 mg dose of Nereus was evaluated across the Motion Series and a 12-month open-label safety study.
Common Adverse Reactions
Adverse reactions reported in geq 5% of subjects and at a higher frequency than placebo include:
- Somnolence: 6% (85 mg) to 12% (170 mg).
- Headache: 7% (85 mg) to 10% (170 mg).
- Fatigue: 6% (85 mg) to 8% (170 mg).
Warnings and Precautions
- Impairment of Alertness: Nereus may impair mental/physical abilities required for driving or operating machinery[36]. This effect is exacerbated by alcohol, CNS depressants, and strong CYP3A4 inhibitors[37].
- Severe Renal/Hepatic Impairment: Avoid use in patients with severe renal impairment (eGFR leq 29 mL/min/1.73m²) or any degree of hepatic impairment (Child-Pugh Class A to C)[38].
Dosing and Administration
Clinical efficacy is highly dependent on timing and dietary state.
- Standard Regimen: A single oral dose of 85 mg or 170 mg[39].
- Timing: Administer approximately 60 minutes before the event expected to cause motion-induced vomiting[40].
- Dietary Status: Administer on an empty stomach (at least 1 hour prior to or 2 hours after a full meal). Food increases C_{max} and AUC significantly, which may alter the safety profile and delay onset.
- Cumulative Limits: Safety for more than 90 total doses has not been established.
Drug Interactions and Pharmacokinetics
Tradipitant is primarily metabolized by hepatic enzymes, introducing potential drug-drug interaction (DDI) risks.
- Metabolism: Tradipitant is a CYP3A4 substrate.
- CYP3A4 Inhibitors: Strong inhibitors (e.g., ketoconazole, clarithromycin) can increase tradipitant exposure and the risk of adverse reactions.
- Half-life: The mean elimination half-life is approximately 34 hours.
- Protein Binding: High plasma protein binding (96% to >99%).
Use in Specific Populations
- Pregnancy: Data are insufficient to inform drug-associated risks. Animal studies at MRHD-equivalent doses showed no adverse developmental effects.
- Lactation: Tradipitant is present in rat milk. Monitor breastfed infants for somnolence.
- Pediatrics: Safety and effectiveness not established in patients under 18.
- Geriatric Use: No overall differences in safety or effectiveness were observed between subjects geq 65 and younger adults, though individual sensitivity cannot be ruled out.
FAQ:
-
When will Nereus be available in pharmacies?
Following its FDA approval on December 30, 2025, Vanda Pharmaceuticals expects to make Nereus commercially available in the United States in the first half of 2026. Patients will require a valid prescription from a healthcare provider to access the medication.
-
How much will Nereus cost?
The official list price (WAC) has not yet been released by the manufacturer. However, as a first-in-class prescription therapy, it is expected to be priced as a premium alternative to over-the-counter options. Most patients with commercial insurance will likely have access through co-pay assistance programs once the drug is added to insurance formularies.
-
Is Nereus available in India?
Currently, Nereus is not yet approved or available in India. While it has received U.S. FDA clearance, it must undergo a separate regulatory review by the Central Drugs Standard Control Organisation (CDSCO) before it can be sold in Indian pharmacies. This process typically takes 12–24 months following a U.S. launch.
-
How should I take Nereus for the best results?
For maximum effectiveness, you must take a single capsule (85 mg or 170 mg) 60 minutes before your travel event. It is critical to take the dose on an empty stomach (at least 1 hour before or 2 hours after a meal), as food can significantly delay how quickly the drug starts working.
-
Can I drive or operate machinery after taking it?
You should exercise extreme caution. The most common side effect is somnolence (drowsiness), affecting up to 12% of patients. It is recommended that you do not drive or operate heavy machinery until you know how Nereus affects you, especially if you have also consumed alcohol or other medications that cause sleepiness.
Conclusion: Forward-Looking Impact
The approval of Nereus represents a scientific and regulatory milestone that ends a 40-year drought in motion sickness research. By blocking the NK-1 receptor and substance P signaling, Vanda Pharmaceuticals has introduced a targeted approach that prioritizes cognitive clarity—a critical factor for military personnel, naval crews, and aviation professionals.
Beyond motion sickness, the clinical success of tradipitant paves the way for expanding indications. Vanda is currently advancing the drug in Phase 3 trials for gastroparesis and as an adjunctive therapy to mitigate the gastrointestinal side effects of GLP-1 receptor agonists. For the millions of travelers affected by motion-induced emesis, Nereus provides a long-awaited prescription option that aligns modern neuropharmacology with the demands of active travel.
Executive Summary for HCPs
- Drug Name: Nereus (tradipitant).
- Indication: Prevention of motion-induced vomiting in adults.
- Mechanism: NK-1 receptor antagonist.
- Key Efficacy: Significant reduction in vomiting incidence (p < 0.0001) with N=681 total subjects across two pivotal trials.
- Main Concern: Somnolence and CNS depression, exacerbated by CYP3A4 inhibitors and alcohol.
- Clinical Pearl: Must be taken on an empty stomach exactly 60 minutes prior to travel.
Disclaimer: This article is for informational purposes only. Healthcare professionals should consult the full prescribing information for NEREUS™ before prescribing.
References:
- Nereus Prescribing Information – Vanda Assets. (2025). Highlights of Prescribing Information: Indications and Usage.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 1: Indications and Usage — Prevention of vomiting induced by motion in adults.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 2.1: Recommended Dosage and Administration — 85 mg or 170 mg single oral dose.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 2.1: Timing — Administer approximately 60 minutes before a motion event.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 2.1: Dietary Requirements — Administer on an empty stomach (1 hour before or 2 hours after a meal).
- Nereus Prescribing Information – Vanda Assets. (2025). Section 2.1: Dosing Limits — Maximum dosage in a 24-hour period is a single dose.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 2.1: Safety Duration — Safety for more than 90 doses has not been established.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 3: Dosage Forms and Strengths — 85 mg white/black opaque capsules.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 4: Contraindications — None.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 5.1: Warnings — Impairment of mental/physical abilities for driving and machinery.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 5.1: Precautions — Increased impairment with CNS depressants and strong CYP3A4 inhibitors.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 6.1: Adverse Reactions — Common reactions (≥5%) include somnolence, headache, and fatigue.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 6.1: Table 1 — Adverse Reaction Incidence Rates (85 mg vs. 170 mg vs. Placebo).
- Nereus Prescribing Information – Vanda Assets. (2025). Section 6.1: Long-term Exposure — 12-month open-label study data (N=382).
- Nereus Prescribing Information – Vanda Assets. (2025). Section 7.1: Drug Interactions — Tradipitant as a CYP3A4 substrate; effects of inhibitors.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 8.1: Pregnancy — Risk summary and animal developmental data.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 8.2: Lactation — Monitoring breastfed infants for somnolence.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 8.4: Pediatric Use — Safety and effectiveness not established.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 8.5: Geriatric Use — Analysis of subjects ≥65 years of age.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 8.6: Renal Impairment — Avoid use in severe impairment (eGFR ≤ 29 mL/min/1.73m²).
- Nereus Prescribing Information – Vanda Assets. (2025). Section 8.7: Hepatic Impairment — Avoid in all degrees of impairment (Child-Pugh A to C).
- Nereus Prescribing Information – Vanda Assets. (2025). Section 11: Description — Chemical structure and molecular formula (C28H16ClF6N5O).
- Nereus Prescribing Information – Vanda Assets. (2025). Section 11: Inactive Ingredients — Croscarmellose sodium, lactose monohydrate, etc..
- Nereus Prescribing Information – Vanda Assets. (2025). Section 12.1: Mechanism of Action — Selective, high-affinity human NK-1 receptor antagonism.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 12.2: Pharmacodynamics — Frontal cortex NK-1 receptor occupancy (up to 93%).
- Nereus Prescribing Information – Vanda Assets. (2025). Section 12.2: Cardiac Electrophysiology — Lack of clinically significant QTc prolongation at 170 mg.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 12.3: Pharmacokinetics — Absorption, Tmax, and 34-hour elimination half-life.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 12.3: Food Effect — 4.7-fold to 6.9-fold increase in Cmax with high-fat meals.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 12.3: Metabolism — Involvement of CYP3A4, CYP2C19, and UGT enzymes.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 13.1: Nonclinical Toxicology — Carcinogenesis, Mutagenesis, and Impairment of Fertility.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 14: Clinical Studies — Methodology for Study 1 (Motion Syros) and Study 2 (Motion Serifos).
- Nereus Prescribing Information – Vanda Assets. (2025). Section 14: Efficacy Results — Table 2: Percentage of subjects with vomiting.
- Nereus Prescribing Information – Vanda Assets. (2025). Section 16: How Supplied/Storage and Handling — 36-count HDPE bottles.
- Comprehensive Evaluation of Nereus (Tradipitant): A New Paradigm in Management of Motion-Induced Emesis. (2025). Regulatory and Historical Context.
- Comprehensive Evaluation of Nereus (Tradipitant). (2025). Comparison to Heritage Therapies and Molecular Architecture.
- Comprehensive Evaluation of Nereus (Tradipitant). (2025). Clinical Trial Program: The Motion Series (Motion Syros and Serifos).
- Comprehensive Evaluation of Nereus (Tradipitant). (2025). Legal and Regulatory “Off-ramp” Strategy.
- Vanda Pharmaceuticals Press Release. (Dec 30, 2025). FDA Approval of NEREUS™ for Prevention of Vomiting Induced by Motion.
- Vanda Pharmaceuticals Press Release. (Dec 30, 2025). Clinical Significance: First new Treatment in 40 Years.
- Vanda Pharmaceuticals Press Release. (Dec 30, 2025). Market Impact and Pipeline Indications (Gastroparesis/GLP-1 Adherence).
