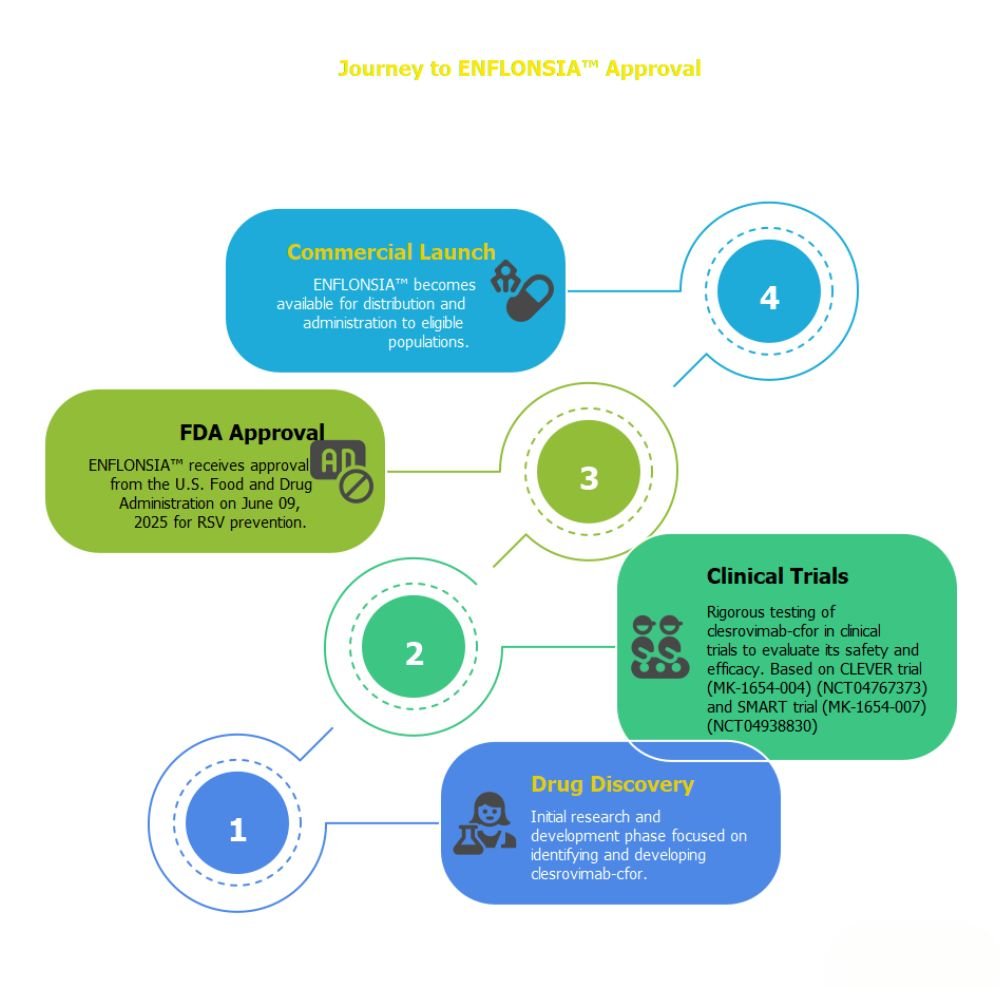
The U.S. Food and Drug Administration (FDA) has approved ENFLONSIA (clesrovimab-cfor), a groundbreaking preventive monoclonal antibody developed by Merck (MSD outside the U.S. and Canada). This single-dose injectable therapy offers seasonal protection against Respiratory Syncytial Virus (RSV) — a major cause of hospitalization in infants. Its approval, announced on June 9, 2025, marks a significant advancement in pediatric respiratory care.
ENFLONSIA at a Glance
An interactive guide to the new RSV treatment.
What is RSV, and why is it a Concern?
Respiratory Syncytial Virus (RSV) is a common seasonal virus that becomes reason of infections in the lungs and respiratory tract. While adults usually experience mild symptoms, infants, especially preterm and those with underlying conditions, are at a high risk of developing serious complications like bronchiolitis and pneumonia.
- RSV is the leading cause of infant hospitalization in the U.S.
- Around 20 lakh children under 5 are treated for RSV in the U.S. annually (CDC).
Also Read: Tryptyr – A Breakthrough in Dry Eye Disease Treatment
Drug Profile: ENFLONSIA at a Glance
| Parameter | Details |
|---|---|
| Drug Name | ENFLONSIA |
| Generic Name | Clesrovimab-cfor |
| Manufacturer | Merck (MSD) |
| Approval Date | June 9, 2025 |
| Indication | RSV prevention in neonates & infants |
| Route of Administration | Intramuscular (IM) injection |
| Dose | Single 105 mg regardless of weight |
| Protection Duration | Up to 5 months (typical RSV season) |
Mechanism of Action of Enflonsia
ENFLONSIA is a long-acting human IgG1 monoclonal antibody designed to provide direct, rapid, and durable immunity against RSV. Once administered, it circulates in the bloodstream, neutralizing the virus and preventing it from infecting lung tissues.
Key Feature: Single-dose administration of 105 mg IM, not weight-dependent — simplifying use in clinical settings.
Clinical Significance
- First-ever RSV preventive option with fixed dosing for all infants.
- Targets a broad range of disease severity.
- Provides coverage throughout an entire RSV season.
Timeline for Availability
Merck has announced that ENFLONSIA™ will be available before the 2025-2026 RSV season, with ordering starting in July 2025.
The Advisory Committee on Immunization Practices (ACIP) is expected to issue its recommendations shortly after.
Clinical Trials: Evidence at a Glance
CLEVER Trial (NCT04767373)
| Aspect | Details |
| Trial Design | Phase 2b/3, randomized, double-blind, placebo-controlled |
| Population | 3,614 infants: 2,411 (ENFLONSIA), 1,203 (Placebo) |
| Primary Outcome | Reduction in RSV-related medically attended LRI (MALRI) |
| Key Result | 60.5% reduction in MALRI incidence (p<0.001) |
| Hospitalization Impact | 84.3% reduction in RSV hospitalizations (p<0.001) |
SMART Trial (NCT04938830)
| Aspect | ENFLONSIA | Palivizumab |
| Participants | 446 | 450 |
| Hospitalizations (%) | 1.3% | 1.5% |
| MALRI (%) | 3.6% | 2.9% |
| Safety Profile | Comparable | Comparable |
Advantages Over Palivizumab
| Feature | ENFLONSIA | Palivizumab |
| Dosing | Single 105 mg IM | Monthly |
| Weight-Based? | No | Yes |
| Hospitalization Reduction | 84.3% | 55% |
| Administration | Easy, one-time | Repeated (monthly) |
| Cost-effectiveness | Likely better | Higher due to dosing |
Dosage and Administration
- Standard Dose: 105 mg/0.7 mL IM
- Infants born during RSV season: Administer at birth
- Infants born before RSV season: Administer just prior to RSV season
- Post-surgery (cardiac): One additional dose after cardiopulmonary bypass
Safety and Side Effects
Most Common (CLEVER Trial):
| Adverse Reaction | ENFLONSIA™ | Placebo |
| Injection-site erythema | 3.8% | 3.3% |
| Injection-site swelling | 2.7% | 2.6% |
| Rash | 2.3% | 1.9% |
- Severity: ≥97% were Grade 1 (mild) or Grade 2 (moderate)
- Concomitant vaccine safety: No significant safety interactions observed
Contraindications:
- History of hypersensitivity or anaphylaxis to any component.
Expert Viewpoint on Enflonsia
“ENFLONSIA’s one-size-fits-all dose, strong efficacy in clinical trials, and better convenience profile over palivizumab make it a game changer in RSV prevention.” — Dr. Anisha Gupta, Neonatologist.
FAQ
Can ENFLONSIA be co-administered with routine infant vaccinations?
Yes, clinical trial data and pharmacovigilance monitoring suggest that ENFLONSIA does not interfere with the safety or efficacy of routine pediatric vaccines such as DTP, MMR, or pneumococcal vaccines. However, providers are advised to administer injections at separate anatomical sites when given concurrently.
How is ENFLONSIA stored and transported in clinical settings?
ENFLONSIA is supplied as a pre-filled syringe and should be stored at 2°C to 8°C (36°F to 46°F). It must not be frozen. Healthcare providers must ensure the cold chain is maintained during transport to clinics and hospitals, similar to routine vaccine handling.
Is ENFLONSIA suitable for premature infants or those with congenital conditions?
Yes, one of the key indications for ENFLONSIA includes preterm infants and those with chronic lung disease or congenital heart disease. Clinical studies have included such subpopulations and shown statistically significant protection with a favorable safety profile.
What are the prescribing considerations for ENFLONSIA in low-resource or rural settings?
Due to its single fixed-dose and non-weight-based administration, ENFLONSIA is particularly suitable for outreach programs, rural clinics, and low-resource pediatric care. It reduces the need for weight calculation, multiple visits, or dose adjustments, simplifying RSV prevention logistics.
Can ENFLONSIA be used in subsequent RSV seasons, or is it a one-time intervention?
ENFLONSIA is indicated for use during each RSV season in eligible infants. For newborns born just before or during the RSV season, annual administration may be needed depending on their age and clinical risk profile in the following year. Physicians will determine eligibility based on updated ACIP guidelines.
Summary
ENFLONSIA emerges as a clinically significant and operationally simplified solution for RSV prevention in infants. Its approval is a testament to how modern monoclonal antibody technology can protect vulnerable populations without complex dosing protocols.
This breakthrough not only elevates clinical outcomes but also streamlines hospital logistics, offering hope to healthcare systems and families alike.
Stay tuned for ACIP recommendations and real-world data as ENFLONSIA™ rolls out in the upcoming RSV season.
References
- U.S. FDA. Press Release on ENFLONSIA Approval
- CDC. RSV Information for Health Professionals
- ClinicalTrials.gov: NCT04767373 (CLEVER)
- ClinicalTrials.gov: NCT04938830 (SMART)