Schedule X Drugs List [2025 Update] – Uses & Side Effects
![Schedule X Drugs List [2025 Update] - Uses & Side Effects schedule x drugs list](https://laafon.com/wp-content/uploads/2024/11/1-768x402.jpg)
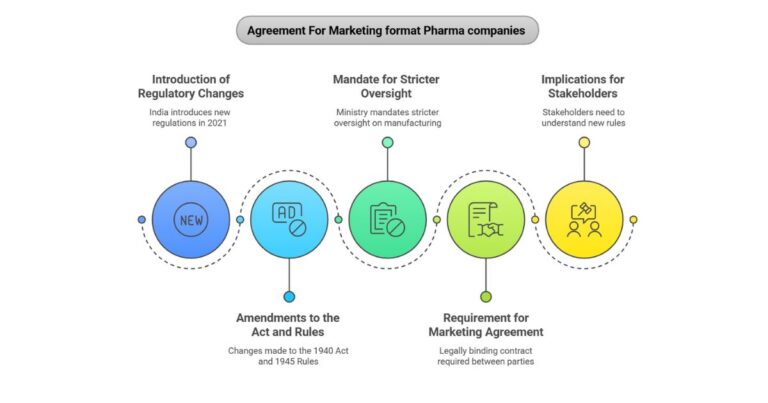
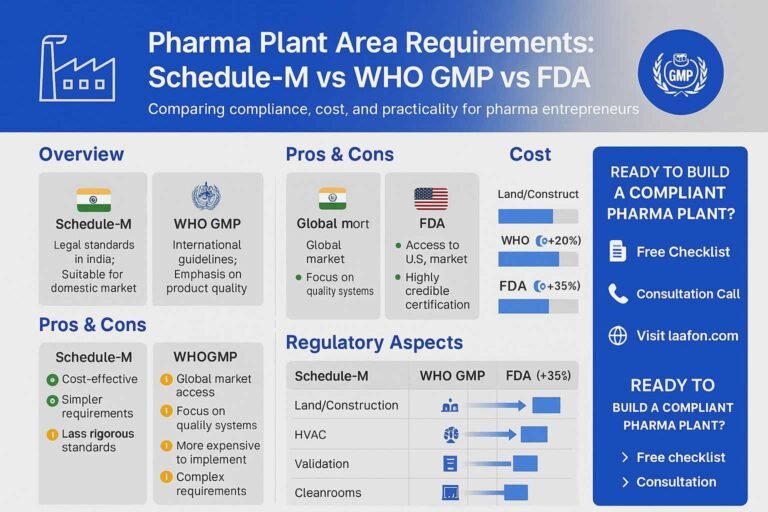
The control of high-risk medication is paramount to public health, and in India, this responsibility falls under the rigorous framework of Schedule X of the Drugs and Cosmetics Rules, 1945. As of late 2025, this classification represents the pinnacle of…