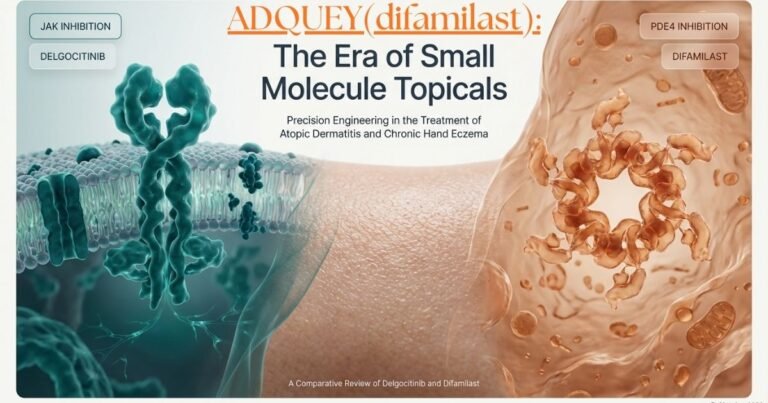
Bysanti FDA Approval: Treatment for Schizophrenia & Bipolar I
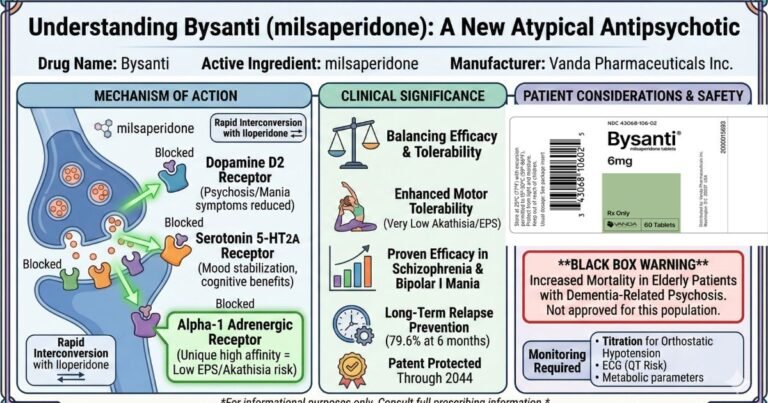
The formal approval of Bysanti (milsaperidone) by the United States Food and Drug Administration (FDA) on February 20, 2026, signifies a sophisticated regulatory and pharmacological evolution within the atypical antipsychotic class. Indicated for the treatment of schizophrenia and the acute…