Yartemlea (narsoplimab-wuug) FDA Approval: First-in-Class Survival Benefit for TA-TMA
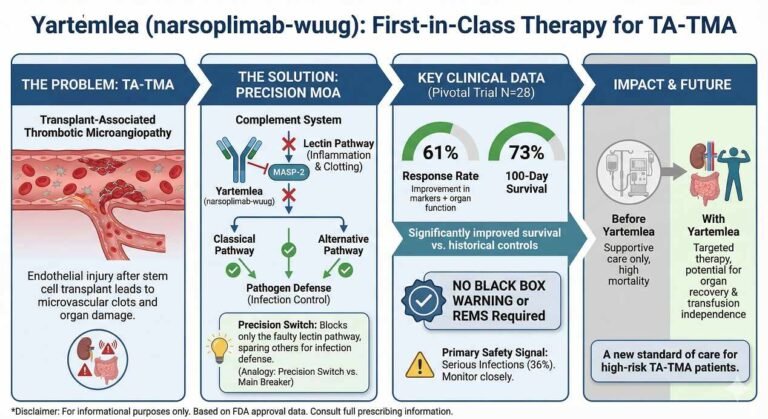
On December 23, 2025, the U.S. Food and Drug Administration (FDA) approved Yartemlea (narsoplimab-wuug), a novel MASP-2 inhibitor developed by Omeros Corporation. This marks a significant regulatory milestone as Yartemlea is the first and only therapeutic indicated for the treatment…
