Breast cancer remains one of the most prevalent and challenging cancers worldwide, with over 300,000 new cases diagnosed annually in the United States alone [4] [10]. Among these, hormone receptor-positive (HR+), and HER2-negative breast cancer accounts for approximately 70% of cases [4] [10]. Despite advancements in endocrine therapies and chemotherapy, many patients face disease progression, highlighting the urgent need for innovative treatments. Enter Datroway (datopotamab deruxtecan), a groundbreaking TROP2-directed antibody-drug conjugate (ADC) that has recently gained FDA approval for treating unresectable or metastatic HR+, HER2-negative breast cancer [3] [5].
This blog post delves into Datroway’s science, efficacy, safety, and future potential, providing a comprehensive resource for patients, caregivers, and healthcare professionals.
Table of Contents
Datroway Development and Collaborative Innovation
Datroway is the result of a strategic collaboration between AstraZeneca and Daiichi Sankyo, leveraging Daiichi’s proprietary DXd ADC Technology. This partnership, initiated in July 2020, builds on their prior success with Enhertu (trastuzumab deruxtecan), another ADC targeting HER2-low breast cancer [2] [13].
- ADC Design: Combines a humanized anti-TROP2 monoclonal antibody with a topoisomerase I inhibitor payload (deruxtecan) via cleavable tetrapeptide linkers.
- Global Pipeline: Datroway is one of six DXd ADCs in Daiichi Sankyo’s oncology pipeline and a cornerstone of AstraZeneca’s ADC platform
What is Datroway?
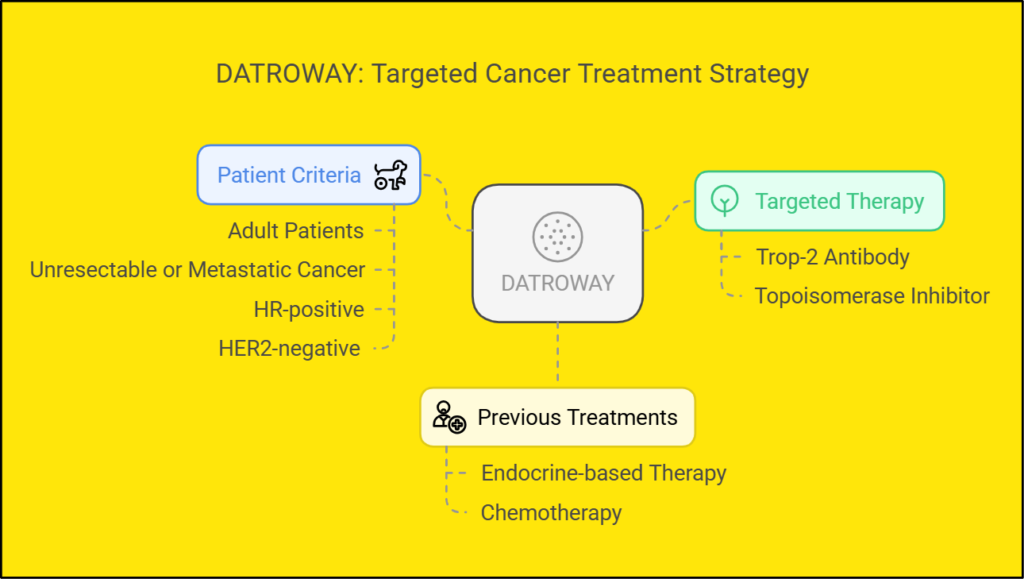
Datroway (datopotamab deruxtecan-dlnk) is an FDA-approved antibody-drug conjugate that targets TROP2-expressing cancer cells. It consists of a humanized anti-TROP2 antibody linked to deruxtecan (DXd), a topoisomerase I inhibitor. FDA approved January 17, 2025, for unresectable or metastatic HR+/HER2- breast cancer following prior therapy.
Mechanism of Action: DATOPOTAMAB DERUXTECAN
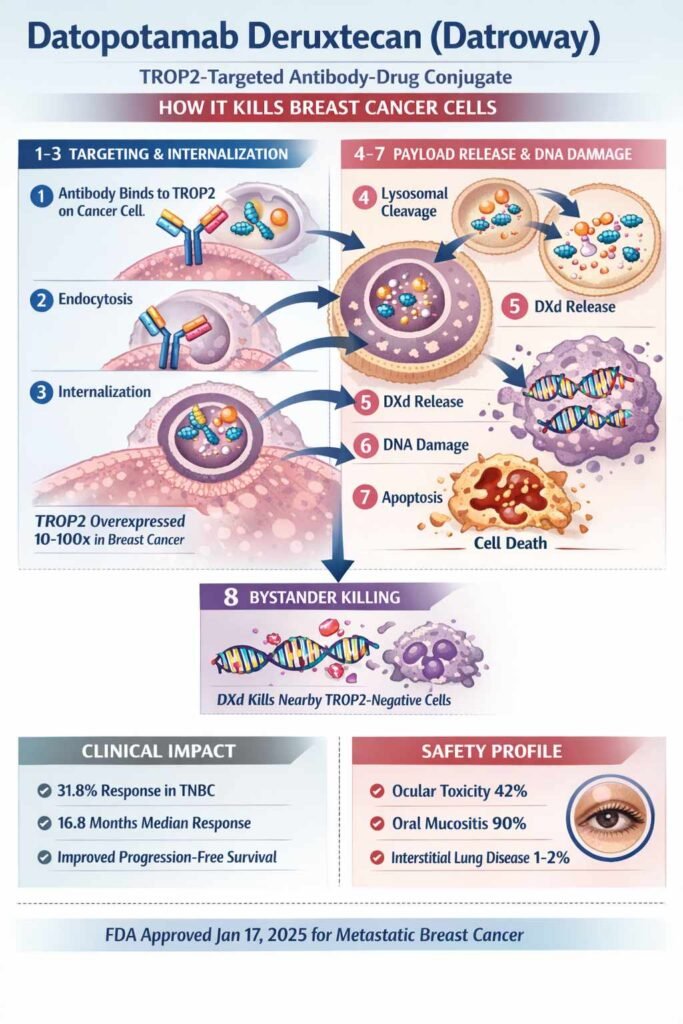
Datopotamab deruxtecan (Datroway) is a TROP2-targeted antibody-drug conjugate (ADC) that works through an eight-step mechanism to kill breast cancer cells[17].
Step 1-3: Targeting and Internalization
The drug’s humanized antibody binds specifically to TROP2, a protein highly overexpressed in hormone receptor-positive (HR+) and triple-negative breast cancer (TNBC). Unlike healthy tissues, cancer cells express TROP2 at 10-100 times higher levels, providing therapeutic selectivity. Upon binding, the complex undergoes rapid clathrin-mediated endocytosis, with 70% internalized by 24 hours. This efficient internalization delivers the cytotoxic payload directly into tumor cells[16, 17].
Step 4-7: Payload Release and DNA Damage
Inside lysosomes, cathepsin proteases cleave the plasma-stable tetrapeptide linker, releasing deruxtecan (DXd)—a topoisomerase I inhibitor 100-1,000 times more potent than conventional chemotherapy. DXd stabilizes topoisomerase I-DNA complexes, preventing DNA religation and creating persistent double-stranded breaks. This triggers cell cycle arrest within 24 hours and apoptosis by 48 hours[17]..
Step 8: Bystander Killing Effect
A unique advantage: DXd’s membrane-permeable properties allow it to enter neighboring TROP2-negative cells, killing heterogeneous tumor populations more effectively than traditional ADCs[18, 17].
Clinical Impact
TROPION-Breast01 trials demonstrated 31.8% response rates in TNBC, 79.5% disease control, and 16.8 months median response duration. TROPION-Breast02 (October 2025) showed datopotamab nearly doubled progression-free survival compared to chemotherapy in metastatic TNBC.
Safety Profile
Mechanism-based toxicities are manageable: ocular surface toxicity (42%) and oral mucositis (90%) reflect topoisomerase I and TROP2 expression in these tissues. Serious interstitial lung disease occurs in only 1-2% of patients.
This selective, multi-target mechanism explains why the FDA approved datopotamab for metastatic breast cancer on January 17, 2025.
FDA Approval and Clinical Trials
TROPION-Breast01 Trial
The FDA approval of Datroway on January 17, 2025, was based on the results of the TROPION-Breast01 phase III trial [3] [5].
Key Findings:
- Progression-Free Survival (PFS): Median PFS was 6.9 months for Datroway vs. 4.9 months for chemotherapy (HR: 0.63; p < 0.0001) [3] [9].
- Overall Survival (OS): Median OS was 18.6 months for Datroway vs. 18.3 months for chemotherapy (HR: 1.01; not statistically significant)[3] [5].
- Objective Response Rate (ORR): Confirmed ORR was 36% for Datroway vs. 23% for chemotherapy[3] [9].
Patient Population:
- Adults with unresectable or metastatic HR+, HER2-negative breast cancer.
- Patients who had received prior endocrine therapy and 1-2 lines of chemotherapy[3] [5].
Efficacy and Safety Profile
Efficacy Highlights
- 37% Reduction in Risk of Disease Progression: Datroway significantly outperformed chemotherapy in delaying disease progression [4] [11].
- Durable Responses: The median duration of response was 6.7 months for Datroway vs. 5.7 months for chemotherapy [9][3].
Safety and Side Effects
While Datroway is generally well-tolerated, some side effects were observed:
Expert Opinions and Clinical Perspectives
- Dr. Aditya Bardia (TROPION-Breast01 Principal Investigator):
“Datroway represents a paradigm shift, offering a chemotherapy-sparing option with targeted efficacy.” [2] [11]. - Dr. Natasha Hunter (Fred Hutch Cancer Center):
“ADCs like Datroway are the future, but sequencing them remains a challenge.” [1] [5]
Datroway Use Risks in Specific Populations
Pregnancy and Potential Risk
According to the data provided by the FDA, Datroway may harm an unborn baby if given to a pregnant woman. One of its main components, DXd, affects fast-growing cells, which means it could impact a developing baby.
Since no studies have been done on pregnant women using Datroway, there is no clear data on how risky it is. However, doctors strongly advise against using this medicine during pregnancy due to the potential harm it may cause to the baby.
Pregnancy Risks in the General Population
In the U.S., the natural risk of birth defects in recognized pregnancies is estimated to be 2-4%, while the risk of miscarriage is between 15-20%. This means that even without medications, these risks exist, but using a drug like Datroway could increase the danger.
Datopotamab Deruxtecan Animal Testing Data
So far, there have been no animal studies to determine how Datroway affects pregnancy or fetal development. Because of this lack of data, medical professionals rely on the drug’s known effects on cell growth to predict its risks.
Therefore, If you are pregnant or planning to become pregnant, talk to your doctor about the risks before considering Datroway.
Common Adverse Reactions (≥20%):
Serious Adverse Reactions:
- Interstitial Lung Disease (ILD): Occurred in 4.2% of patients, with 0.5% experiencing Grade 3-4 ILD [11].
- Ocular Adverse Reactions: Dry eye and keratitis were the most common, leading to treatment discontinuation in 0.8% of patients[11].
Management Strategies:
- ILD: Monitor for respiratory symptoms and initiate corticosteroids if needed[11].
- Ocular Reactions: Use preservative-free lubricant eye drops and refer patients to an eye care professional[11].
Comparison with Other Therapies
Datroway vs. Chemotherapy
| Parameter | Datroway | Chemotherapy |
|---|---|---|
| Median PFS | 6.9 months | 4.9 months |
| Median OS | 18.6 months | 18.3 months |
| ORR | 36% | 23% |
| Common Side Effects | Stomatitis, nausea, fatigue | Nausea, fatigue, alopecia |
Key Findings after Clinical Trials of Datroway:
| Parameter | Datroway Group | Standard Chemotherapy |
|---|---|---|
| Progression-Free Survival (PFS) | 6.9 months | 4.9 months |
| Overall Survival (OS) | 18.6 months | 18.3 months |
| Confirmed Objective Response Rate (ORR) | 36% | 23% |
| Complete Response Rate | 0.5% | 0% |
| Median Duration of Response (DOR) | 6.7 months | 5.7 months |
Datroway vs. Enhertu
Enhertu (trastuzumab deruxtecan), another ADC, is approved for HER2-low breast cancer. While both drugs share a similar mechanism, Datroway targets TROP2, making it suitable for HER2-negative patients[7] [10].
Comparison with Other TROP2-Directed Therapies
| Parameter | Datroway | Trodelvy (Sacituzumab Govitecan) |
|---|---|---|
| Target | TROP2 | TROP2 |
| Indication | HR+/HER2- MBC | TNBC |
| Median PFS (vs. chemo) | 6.9 months | 5.6 months |
| ILD Risk | 4.2% | 3% |
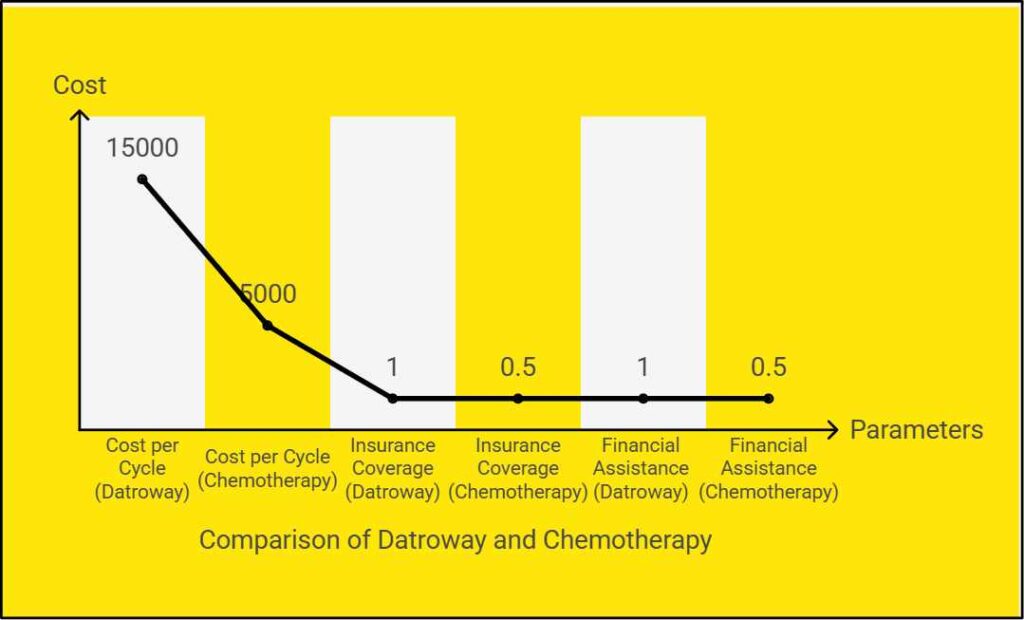
| Cost per Cycle | ~$15,000 | ~$13,000 |
Future Directions
Ongoing Trials
- TROPION-Breast02: Evaluating Datroway in triple-negative breast cancer (TNBC)[4].
- TROPION-Lung01: Investigating Datroway in non-small cell lung cancer (NSCLC)[3].
Potential for Earlier Use
Researchers are exploring Datroway’s efficacy in earlier lines of therapy, potentially replacing chemotherapy in certain settings[4] [11].
Patient-Centric Insights about Datroway
Cost and Accessibility
As of February 2025, Datroway costs approximately $15,000 per cycle in the U.S., and most insurance plans cover the treatment[11]. Patients are encouraged to consult their healthcare providers for financial assistance programs.
- Price: ~$15,000 per cycle in the U.S.
- Insurance Coverage: Most private plans and Medicare Part B cover Datroway.
- Patient Assistance: AstraZeneca’s Patient Assistance Program offers financial support.
Patient Support Programs
- AstraZeneca Patient Assistance Program: Offers financial support for eligible patients[11].
- Living Beyond Breast Cancer: Provides resources and support for metastatic breast cancer patients[4].
Patient Eligibility and Biomarker Insights
Who Qualifies?
- Adults with unresectable/metastatic HR+/HER2-negative breast cancer (IHC 0, 1+, or 2+/ISH-).
- Prior therapies: At least one endocrine-based regimen and 1–2 lines of chemotherapy [2] [5].
TROP2 as a Biomarker
- TROP2 Overexpression: Found in ~70% of HR+/HER2- breast cancers, making it an ideal therapeutic target [2] [15].
Recommended Dosage of Datroway
- Dose: 6 mg/kg intravenously every 21 days (maximum 540 mg for patients ≥90 kg)[5] [11].
- Infusion Protocol:
- First dose: Administered over 90 minutes.
- Subsequent doses: Reduced to 30 minutes if tolerated
Premedication and Supportive Care [Important]
- Premedication: Antihistamines, antipyretics, and antiemetics to mitigate infusion-related reactions4.
- Ocular Care: Preservative-free lubricant eye drops 4x/day; avoid contact lenses [4] [11].
Global Access and Equity of Datorway
- U.S. Availability: Launched on February 1, 2025; accessible via Datroway4u.com [13].
- Low-Income Countries: AstraZeneca’s Global Access Program aims to subsidize costs in underserved regions [2].
- In India, currently, Datroway is not available, however, Laafon Galaxy Pharmaceuticals is in the process of getting distribution rights of Datroway for India. Both companies are in engagement for this.
Emergency Support:
Call your healthcare provider for medical advice about side effects. You may report side effects to Daiichi Sankyo at 1-877-437-7763 or to the FDA at 1-800-332-1088.
Conclusion
Datroway (datopotamab deruxtecan) represents a significant advancement in the treatment of HR+, HER2-negative breast cancer. With its targeted mechanism, proven efficacy, and manageable safety profile, it offers hope to patients who have exhausted traditional therapies. As ongoing trials explore its potential in other cancers, Datroway is poised to become a cornerstone of modern oncology.
Staying informed about treatment options like Datroway is crucial for patients and caregivers. Consult your healthcare provider to determine if this therapy is right for you.
FAQs
Who is eligible for Datroway treatment?
Datroway is approved for adults with unresectable or metastatic HR+/HER2-negative breast cancer who have received prior endocrine therapy and at least one line of chemotherapy in the advanced setting 25. Patients must have HER2-negative status confirmed by IHC (0, 1+, or 2+/ISH-) and disease progression after endocrine-based therapies.
What is the cost of Datroway, and is financial assistance available?
U.S. cost: Approximately $15,000 per treatment cycle 8.
Insurance coverage: Most private plans and Medicare Part B cover Datroway.
Patient support: Daiichi Sankyo and AstraZeneca offer copay assistance programs for eligible patients. Contact their support line (1-877-437-7763) or visit Datroway.com for details.
References
- FDA Approves Datopotamab Deruxtecan for Unresectable or Metastatic HR+ Breast Cancer.[3]
- Datroway Approved in the US for HR+ Breast Cancer.[4]
- FDA Approves Datopotamab Deruxtecan-drink for Unresectable or Metastatic Breast Cancer.[5]
- Breaking Down Cancer Therapies Approved by the FDA in January 2025.[6]
- Enhertu Approved for HER2-Low Breast Cancer. [7]
- Datroway Safety and Efficacy.[9]
- Enhertu’s Role in Breast Cancer Treatment.[10]
- Datroway U.S. Prescribing Information.[11]
- Datroway Clinical Trials and Future Directions. [12]
- EMA’s Positive Opinion on Datroway. [14]
- DATROWAY® (datopotamab deruxtecan-dlnk) approved in the US for patients with previously treated metastatic HR-positive, HER2-negative breast cancer-AstraZeneca(.com)
- Expert Commentary on ADCs [15].
- Datopotamab Deruxtecan, a Novel TROP2-directed Antibody–drug Conjugate, Demonstrates Potent Antitumor Activity by Efficient Drug Delivery to Tumor Cells[PMC]
- Decoding TROP2 in breast cancer: significance, clinical implications, and therapeutic advancements[TROP2]
- Datopotamab deruxtecan, a novel TROP2-tareting antibody-drug conjugate with a topoisomerase I inhibitor payload, shows preclinical activity against primary and metastatic uterine and ovarian TROP2 over-expressing carcinosarcoma.[ScienceDirect]