For those living with the constant uncertainty of hereditary angioedema (HAE), a significant breakthrough has arrived. A new treatment, Andembry® (garadacimab-gxii), was recently approved by the U.S. Food and Drug Administration (FDA) on June 16, 2025, offering a powerful new strategy for routinely preventing the recurrent and debilitating attacks associated with HAE in individuals aged 12 and older.
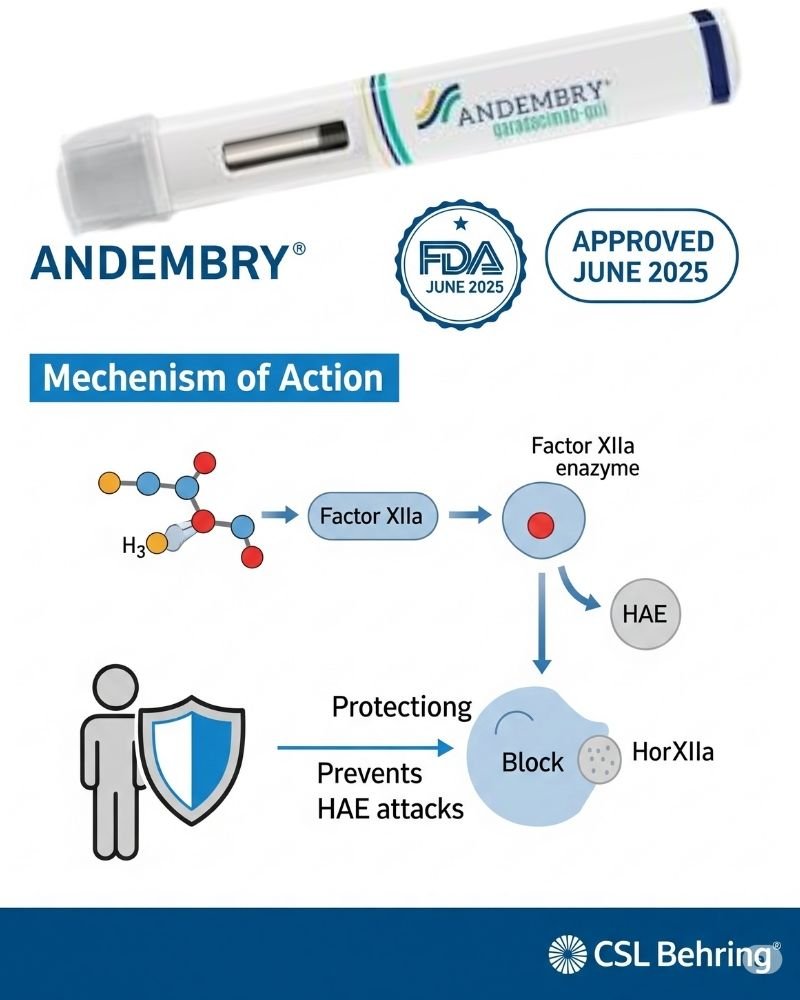
It is developed by CSL Behring, which represents a major step forward in HAE management. It is a fully human recombinant monoclonal antibody that works by targeting Factor XIIa (FXIIa), the very enzyme that initiates the inflammatory chain reaction leading to HAE attacks.
Global Approvals of Andembry
Beyond its recent FDA(US) approval on June 16, 2025, Andembry has quickly gained regulatory acceptance worldwide, highlighting the global need for advanced HAE therapies. Its journey to market includes:
- Australia & UK: First approvals in January 2025.
- Japan & Switzerland: Cleared in February 2025.
- European Union: Received marketing authorization in February 2025, following a positive opinion from the European Medicines Agency (EMA) in December 2024.
Throughout these regions, it is available only by prescription and holds an orphan drug designation in the European Union, a status given to therapies for rare conditions.
Andembry Targets the Source of HAE Attacks
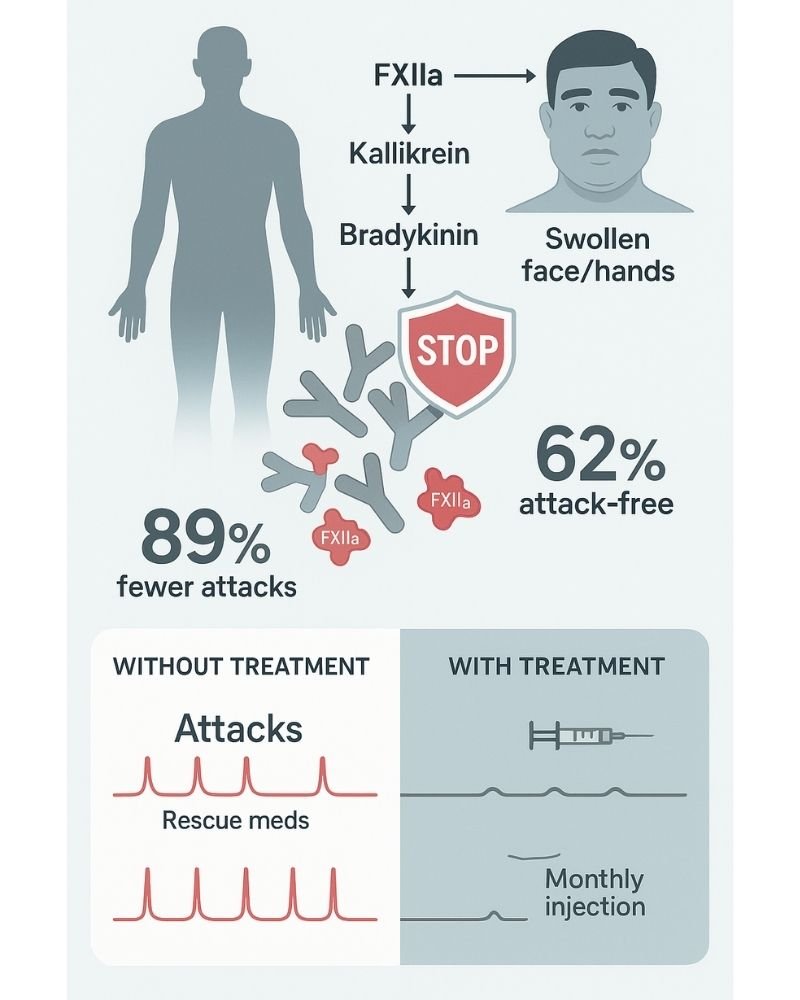
To understand why Andembry is so effective, we need to look at the biology of HAE. The condition is driven by an overproduction of bradykinin, a substance that causes swelling and pain. It zeroes in on activated Factor XII (FXIIa), a critical enzyme at the very top of this cascade. By inhibiting FXIIa, it effectively prevents the downstream generation of kallikrein and bradykinin, stopping attacks before they can even start. A remarkable feature is that this powerful inhibitory effect is concentration-dependent and begins after just the first dose.
Clinical Trials-2: The Foundation- Successful Phase 2 Trials
Andembry’s path to approval was built on a strong clinical foundation. The journey began with a successful randomized, placebo-controlled Phase 2 trial, with results published in The Lancet. This was followed by a long-term, open-label extension study to assess sustained effectiveness and safety.
Published in journals like Lancet Haematology, this extension study confirmed that Andembry provides lasting reductions in HAE attacks and maintains a favorable safety profile over years of use. This robust early data paved the way for the pivotal VANGUARD trial, demonstrating the drug’s promise from the outset.
Clinical Trials-3: The VANGUARD Trial Results
The excitement surrounding Andembry isn’t just hype; it’s supported by compelling data from the pivotal VANGUARD clinical trial. The results, which were both statistically significant (p<0.001) and clinically life-changing, speak for themselves:
- An 89.2% average reduction in the monthly rate of HAE attacks compared to a placebo.
- A 91.2% decrease in the number of attacks that required rescue medication.
- A 93.6% drop in moderate-to-severe attacks.
- An incredible 62% of patients became completely attack-free.
- 74% of patients experienced a reduction in their attack rate of 90% or more.
Administration and Dosage of Andembry
CSL Behring designed Andembry with patient convenience in mind. The dosing schedule is simple and manageable:
- Loading Dose: A one-time initial dose of 400 mg, administered as two separate 200 mg injections.
- Maintenance Dose: A single 200 mg subcutaneous injection once a month.
Patients or their caregivers can perform the injections at home. The approved injection sites are the thigh or abdomen (staying at least 2 cm away from the navel). A caregiver can also administer the injection into the upper arm. For comfort, it’s recommended to let the injector sit at room temperature for 30 minutes before use.
It is available as a sterile, preservative-free solution in two single-dose formats: a 200 mg/1.2 mL autoinjector and a 200 mg/1.2 mL prefilled syringe with a needle safety feature. After use, the injectors must be disposed of in a proper sharps container.
Safety Profile:
The clinical trials showed Andembry to have a clean and predictable safety profile, with no major warnings or contraindications. The most commonly reported side effects included nasopharyngitis (common cold symptoms), abdominal pain, and minor injection site reactions, which occurred in about 14% of patients.
An important note for healthcare providers: It can interfere with certain lab assays, causing transient changes in clotting time tests (aPTT/PT/INR). This is an expected result of the drug’s mechanism and has not been linked to any increased risk of bleeding. While anti-drug antibodies were detected in some participants, they did not impact the drug’s overall safety or effectiveness.
Regarding its use in specific populations:
- Pregnancy: While there is no human data, animal studies showed no evidence of fetal harm.
- Lactation: It is not known if Andembry passes into breast milk; a discussion of benefits and risks with a doctor is recommended.
- Pediatrics & Geriatrics: It has been proven safe and effective for patients 12 and older, with no significant differences observed in older populations.
- Renal Impairment: It is considered safe for patients with mild to moderate renal impairment, though its effects in severe cases are unknown.
Storage and Handling
To maintain its integrity, It should be stored in a refrigerator between 2°C and 8°C (36°F and 46°F). It must be kept in its original carton to protect it from light and should never be frozen or shaken.
Summary:
Andembry is more than just another treatment; it’s a strategic evolution in how we approach hereditary angioedema. By intervening at the very top of the attack cascade, it offers a highly effective prophylactic strategy. Combined with its strong clinical data and a user-friendly monthly, self-injectable schedule, Andembry puts a new level of control back into the hands of patients.
For healthcare providers, HAE advocates, and patients exploring preventative options, Andembry certainly deserves a close look. As it continues to roll out globally, further information on cost, insurance access, and real-world patient experiences will become available.
Product & Manufacturer Information
- Brand Name: ANDEMBRY®
- International Nonproprietary Name (INN): Garadacimab
- Manufacturer: CSL Behring (USA & Germany)
- Distributor: CSL Behring LLC
- More Information: Contact Us
- NDC Codes: 63833-925-01 (Carton), 63833-925-20 (Autoinjector)
Have questions about integrating ANDEMBRY into your treatment plan? Drop them in the comments or connect via our Contact page.
FAQs
What is ANDEMBRY® and what is it used for?
ANDEMBRY® (garadacimab-gxii) is a prescription medication used for the routine prevention of recurrent attacks in people aged 12 years and older with Hereditary Angioedema (HAE). It works by targeting a plasma protein called Factor XIIa, which plays a key role in triggering HAE attacks, thereby interrupting the HAE cascade and helping to prevent swelling episodes. It is currently the only prophylactic therapy for HAE that offers a once-monthly dosing option from the start for all patients.
What will be the price of Andembry in India?
Currently, it is available in India at Rs. 70000/- per vial on several B2C portals.
References:
- Garadacimab-[Wikipedia(https://en.wikipedia.org/wiki/Garadacimab)]
- Hereditary Angioedema-[National Library of Medicines(https://www.ncbi.nlm.nih.gov/books/NBK482266/)]
- CSL312 (Garadacimab) in the Prevention of Hereditary Angioedema Attacks-[Wikipedia(https://www.clinicaltrials.gov/study/NCT04656418)]
- A Study to Investigate CSL312 in Subjects With Hereditary Angioedema (HAE)-[ClinicalTrials.gov(https://www.clinicaltrials.gov/study/NCT03712228)]