Montek-LC Tablet is a widely used combination medication in India’s pharmaceutical market for treating various allergic conditions and supporting asthma management. It combines the anti-inflammatory effects of Montelukast and the antihistaminic action of Levocetirizine. Given its efficacy and safety profile, Montek-LC is a product of reputable manufacturers, with Sun Pharmaceutical Industries Ltd. being a leading producer. This article provides a step-by-step regulatory and clinical guide to Montek-LC Tablet, reviewing its company background, therapeutic benefits, side effects, dosing, cost/ROI, and evidence-based insights for plant owners, distributors, and healthcare professionals.
Montek-LC Tablet: Uses, Side Effects, Composition
Montek-LC Tablet is a prescription medication combining two active substances: Montelukast (10 mg) and Levocetirizine (5 mg). Montelukast is a leukotriene receptor antagonist, while Levocetirizine is a second-generation antihistamine. The tablet is primarily manufactured by Sun Pharmaceutical Industries Ltd, though several other companies in India market it under similar branding and composition.
Also Read: pH meter principle and Working
Company Name, Manufacture, and Approvals
Montek-LC Tablet is most commonly associated with Sun Pharmaceutical Industries Ltd, a USFDA-approved and WHO-GMP-certified manufacturer based in India. Other Indian pharma giants, such as Cipla, Mankind, Alkem, Lupin, and Intas, also produce similar Montelukast + Levocetirizine tablets under their own trademarks. This widespread regulatory approval ensures product reliability and easier procurement for plant owners and distributors.
| Product Name | Main Manufacturer (India) | Regulatory Approval |
|---|---|---|
| Montek-LC Tablet | Sun Pharmaceutical Industries Ltd | USFDA, WHO-GMP |
| Montair-LC | Cipla Ltd | USFDA, WHO-GMP |
| Generic Versions | Alkem, Lupin, Intas, and others | WHO-GMP, Schedule M |
Composition & Technical Mechanism
Ingredients
- Montelukast Sodium (10 mg): A selective leukotriene receptor antagonist, blocks leukotriene-mediated airway inflammation and bronchoconstriction.
- Levocetirizine Hydrochloride (5 mg): A non-sedating antihistamine, competitively blocks H1 receptors, reducing allergic symptoms like sneezing, itching, and runny nose.
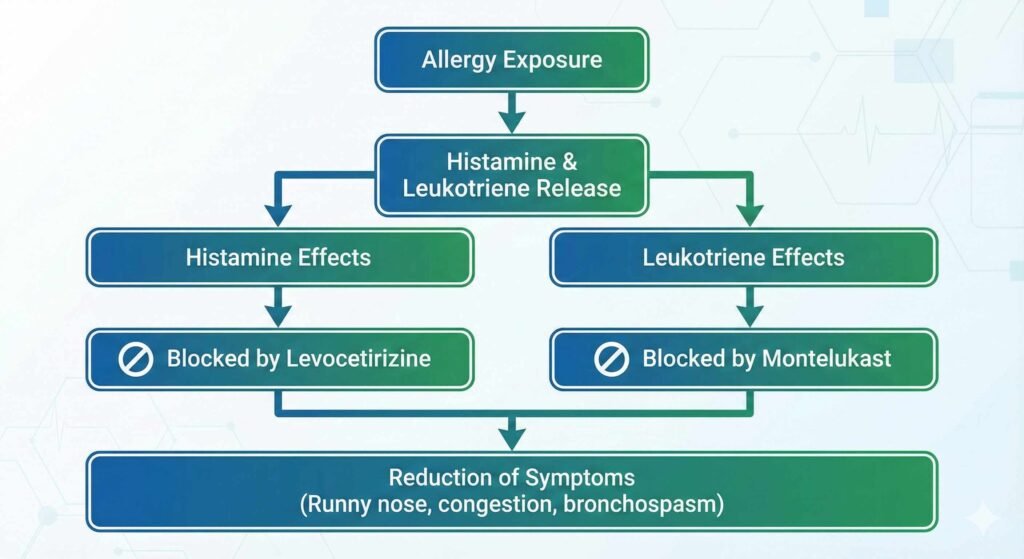
Step-by-Step Mechanism of Action
- Montelukast Inhibition: Prevents leukotrienes from binding to their receptors, reducing inflammation, edema, and bronchoconstriction in allergic and asthmatic conditions.
- Levocetirizine Antagonism: Blocks histamine effects at H1-receptors, mitigating common allergic symptoms (runny nose, itchy eyes, sneezing).
Real-World Example
A patient with seasonal allergic rhinitis presenting with sneezing, watery eyes, and mild asthma receives Montek-LC. Symptoms usually subside within 24 hours of administration, with improved breathing and reduced irritation.
Indications & Uses
Montek-LC Tablet is prescribed for:
- Allergic rhinitis (seasonal and perennial)
- Chronic urticaria (hives)
- Asthma (maintenance, adjunct to standard therapy)
- Sinus congestion and postnasal drip
- Allergic skin conditions (itching, rash)
Montek LC for Dry Cough: Evidence and Guidance
Montelukast (a component of Montek-LC) has shown promise in treating persistent cough, especially in cases where allergy or airway hyper-reactivity is suspected. However, clinical guidelines recommend Montek-LC mainly for cough caused by allergies or asthma, not routine viral coughs. Physicians may prescribe Montek-LC for dry cough only after ruling out infectious and non-allergic causes.
Dosage and Administration
Always swallow the tablet whole, do not crush or chew. Regular dosing at the same time daily is essential for optimal effect.
Side Effects and Safety
Common Side Effects
- Drowsiness, fatigue, headache
- Dry mouth, stomach discomfort, diarrhea
- Skin rash, sleepiness
- Nausea, vomiting, sore throat
Serious/Reactions (Rare)
- Mood changes (depression, anxiety)
- Allergic reactions (swelling, tightening of chest, difficulty breathing)
- Liver enzyme elevation (rare)
Special caution is advised for patients with severe liver or renal impairment, and those with a history of neuropsychiatric symptoms.
Regulatory and Real-World Manufacturing Guidance
Regulatory Standards
- Sun Pharma and most Montek-LC manufacturers are WHO-GMP and, in cases, USFDA certified.
- Tablets marketed in India must comply with Schedule H of the Drugs and Cosmetics Act.
- Batch records, stability testing, composition certificates required during audits.
- Plant owners should focus on traceability, API sourcing purity, and regulatory documentation, especially for export or tender procurement.
Real-World Plant Owner Example
Pharma plants in Baddi or Uttarakhand planning to manufacture Montek-LC must ensure:
- Dedicated production lines for antihistamine-leukotriene tablets
- Validation records and GMP-compliant batch documentation
- Traceable raw materials, periodic quality audits
- Market research for branding under different company names for local, national, and export markets.
Cost, ROI, and Market Insights
| Product (Brand) | Price (per 15 tablets) | Manufacturer |
|---|---|---|
| Montek-LC Tablet | ₹306–₹340 | Sun Pharma |
| Generic equivalents | ₹250–₹330 | Multiple manufacturers |
Substitutes & Cost Comparison
| Brand Name | Price (₹) | Manufacturer |
|---|---|---|
| Montek-LC | 373 | Sun Pharma |
| Zinte-M | 99.00 | Laafon Galaxy Pharmaceuticals |
| Levocet-M | 108.75 | Hetero Labs |
| Montemac-L | 163.00 | Macleods Pharmaceuticals |
Tip: Generics contain the same active ingredients or biosimilars but may differ in binders and dissolution properties of the product. Consult your doctor before switching.
ROI for Plant Owners/Distributors
- High demand due to India’s allergy and asthma prevalence
- Multi-brand licensing enables better market coverage
- Competitive pricing, bulk procurement benefits, and trusted regulatory profile
Also Read: Pharma top 10 companies in India
FAQs
Can I take Montek-LC for a dry cough?
Yes, if the cough is allergy or asthma-related. Avoid self-medication. But do not try self-medication always take the advice of a health professional.
How long does it take to work?
Symptom relief starts in 1-2 hours; full effect may take 1-2 weeks. The results may differ from patient to patient depending on the physical status, age, and severity of the disease.
Is drowsiness common?
Levocetirizine causes mild drowsiness in 10% of users.
Montek LC company name?
Most commonly Sun Pharma; other major Indian pharmaceutical companies also produce Montek-LC branded generics.
Is Montek LC safe for pregnant or breastfeeding women?
Use with caution during pregnancy, not recommended for breastfeeding women unless doctor approves.
Buy Online from Amazon:
Conclusion
Montek-LC Tablet is a robust, evidence-backed solution for allergy and mild asthma management, produced by leading Indian pharma companies with strong regulatory approval. For plant owners, ensuring compliance, multi-brand licensing, and focused regulatory documentation are essential to maximize ROI and market penetration. Healthcare professionals and distributors should advise patients on proper use, monitor for side effects, and avoid misuse. Regular regulatory and GMP audits, coupled with strong sourcing strategies, will ensure consistent product quality and legal compliance.
References:
- Sun Pharma Quality & Manufacturing:[ Source: Sun Pharmaceutical Industries Ltd. Q3 Report 2024-2025.(https://www.reuters.com/business/healthcare-pharmaceuticals/indias-top-drugmaker-sun-pharmas-q3-profit-beats-estimates-strong-local-sales-2025-01-31/)]
- Bronchodilators & Mast Cell Stabilizers(Montelukast)/Drug Today(Year book 2017) Page 346.
- Montelukast Mechanism & Leukotriene Inhibition [Source: National Institutes of Health (NIH). “Montelukast: A Comprehensive Review.”]
- GINA Guidelines for Asthma [Source: Global Initiative for Asthma (GINA). “2023 GINA Report.”]
- Montelukast-Pubchem
- FDA Approval of Montelukast [Source: U.S. Food and Drug Administration (FDA). “Singulair (Montelukast) Label.”]
- Levocetirizine Safety & Minimal Sedation [Source: Church, M.K., & Maurer, M. (2017). British Journal of Clinical Pharmacology.]

