Nimesulide and paracetamol tablets are fixed‑dose combination (FDC) analgesic–antipyretic products widely marketed in India for short‑term relief of acute pain and fever, but nimesulide carries significant hepatotoxic and gastrointestinal (GI) safety concerns and is tightly restricted or banned in many countries. For a pharma entrepreneur, this FDC sits in a clinically useful but high‑scrutiny niche where labeling, promotion, and pharmacovigilance must be handled very carefully.

Overview of Nimesulide and Paracetamol Tablets
Nimesulide and paracetamol tablets are oral FDCs combining a relatively COX‑2–selective NSAID (nimesulide) with a centrally acting analgesic–antipyretic (paracetamol) for short‑term relief of mild to moderate pain and fever. They are commonly used in India for headaches, dental pain, musculoskeletal pain, dysmenorrhoea, and febrile states when rapid symptomatic relief is required.
Because of nimesulide‑associated liver toxicity, multiple regulators—including EMA and several national agencies—have imposed strict duration and dose limits, and the product is banned in many markets outside India. In India, oral nimesulide remains available but under growing scrutiny, including recent recommendations to restrict strength, indications, and vulnerable populations.
Brand profile: Laanim Plus
- Brand name: Laanim Plus
- Generic name: Nimesulide and Paracetamol Tablets
- Composition:
- Nimesulide 100 mg
- Paracetamol 325 mg
- Dosage form: Oral tablets for systemic use
- Therapeutic class:
Composition and mechanism of action
Nimesulide
- Pharmacological class: Non‑steroidal anti‑inflammatory drug; relatively selective COX‑2 inhibitor.
- Mechanism of action:
Paracetamol
- Pharmacological class: Central non‑opioid analgesic and antipyretic.
- Mechanism of action (current understanding):
Synergistic effect of the combination
- Nimesulide primarily targets peripheral inflammatory mediators, while paracetamol acts predominantly in the CNS to reduce pain and temperature set‑point.
- The combination can provide broader and sometimes faster pain relief than either agent alone, but it also accumulates the hepatotoxic risk of both drugs, making patient selection and duration limits critical.
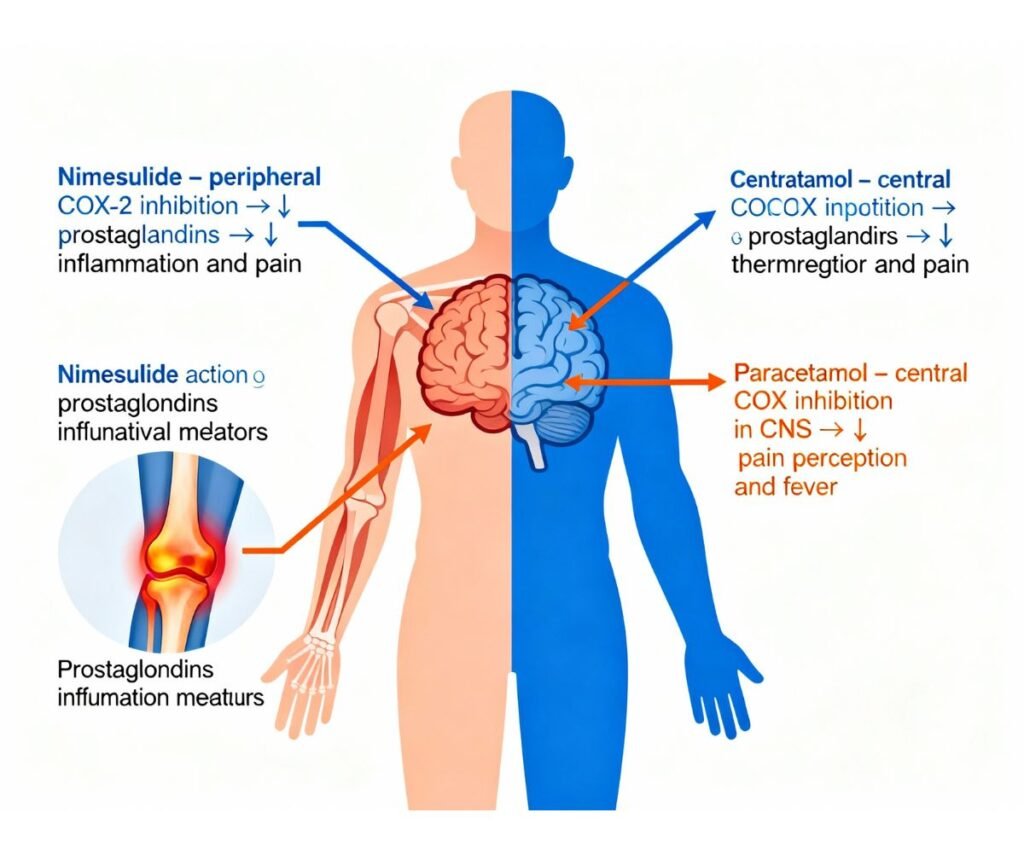
Pharmacokinetics
Nimesulide pharmacokinetics
- Absorption: Rapidly absorbed after oral administration; peak plasma concentrations typically within 1.5–2.5 hours.
- Distribution: Highly protein bound (≈99%), with good penetration into inflamed tissues and synovial fluid.
- Metabolism: Extensively metabolized in the liver, primarily via CYP‑mediated pathways to active and inactive metabolites (e.g., 4‑hydroxynimesulide).
- Elimination half‑life: Approximately 2–5 hours in healthy adults.
- Excretion: Mainly via urine and feces as metabolites.
Paracetamol pharmacokinetics
- Absorption: Rapid and nearly complete absorption; peak levels within 30–60 minutes for immediate‑release tablets.
- Metabolism:
- Elimination half‑life: Around 2–3 hours in healthy adults.
- Excretion: Renal, mainly as conjugated metabolites.
Pharmacodynamics and clinical effects
- Analgesic effect: Reduction in inflammatory pain (nimesulide) and central pain perception (paracetamol) provides effective relief in acute musculoskeletal pain, dental procedures, and dysmenorrhoea.
- Antipyretic effect: Both agents lower fever by acting on hypothalamic thermoregulation, with paracetamol contributing more strongly to the antipyretic profile.
- Onset and duration: Onset is typically within 30–60 minutes, with clinical effect lasting several hours, supporting short‑term symptomatic use rather than chronic therapy.
Indications:
Most Indian brands of nimesulide and paracetamol tablets are approved and promoted for short‑term treatment of acute painful and febrile conditions in adults and adolescents, such as:
- Headache and mild migraine
- Dental pain and post‑extraction pain
- Musculoskeletal pain, including sprain, strain, back pain
- Osteoarthritis and other degenerative joint pain flares
- Primary dysmenorrhoea
- Fever associated with infections (as adjunctive symptomatic treatment)
Use is generally recommended only when monotherapy with a single analgesic/antipyretic is considered insufficient and after assessing patient‑specific risk factors.
Always align final indication wording with your approved package insert and DCGI permission letter.
Contraindications:
Nimesulide and paracetamol tablets are typically contraindicated in:
- Hypersensitivity to nimesulide, paracetamol, other NSAIDs, or excipients
- History of NSAID‑induced bronchospasm, angioedema, or urticaria
- Active or recurrent peptic ulcer, GI bleeding, or perforation
- Severe hepatic impairment, active liver disease, or previous nimesulide‑related hepatotoxicity
- Severe renal impairment
- Severe heart failure or uncontrolled hypertension
- Pregnancy (especially third trimester) and breastfeeding as per many expert and regulator recommendations
- Children below a specified age/weight (commonly below 12 years or <20 kg; confirm with Indian label)
- Alcohol or substance misuse with underlying liver risk
Because of the hepatotoxic profile, many authorities recommend avoiding use in any patient with active hepatic disease, elevated transaminases, or concomitant hepatotoxic drugs.
Dosage, administration, and duration limits
- Typical adult dose:
- Maximum duration (evidence‑based):
Key administration precautions
- Swallow tablet with water after meals to reduce GI irritation.
- Do not exceed prescribed dose or combine with additional paracetamol products, as cumulative daily paracetamol dose should not exceed 3–4 g in adults (often lower in liver disease or low body weight).
- Avoid alcohol and other hepatotoxic drugs during therapy.
- Discontinue immediately and seek medical review if symptoms such as jaundice, intense fatigue, pruritus, dark urine, or right‑upper‑quadrant pain occur.
Adverse effects and risk factors
Common adverse effects
- Gastric pain, nausea, vomiting, dyspepsia, diarrhoea
- Headache, dizziness, somnolence
- Rash, pruritus, mild hypersensitivity reactions
Serious adverse effects
- Hepatotoxicity (core safety concern):
- Nimesulide has been associated with acute hepatitis, cholestatic injury, and rare fulminant hepatic failure requiring transplantation.
- Systematic reviews show increased risk of liver injury compared with some other NSAIDs, leading to suspension or withdrawal in countries such as Ireland, Finland, Spain, and several non‑EU markets.
- Paracetamol adds its own dose‑dependent risk, especially in overdose or chronic alcohol use.
- GI toxicity:
- Renal effects:
- Hypersensitivity:
Key risk factors for toxicity
- Pre‑existing liver disease or elevated LFTs
- Chronic alcohol intake
- Concomitant hepatotoxic drugs (e.g., high‑dose antituberculars, certain antiepileptics)
- Older age, low body weight, dehydration, or multiple comorbidities
- Prior history of NSAID‑induced GI bleeding or ulcers
Drug interactions
Relevant interactions for nimesulide and paracetamol tablets include:
- Anticoagulants (e.g., warfarin), antiplatelets
- Increased risk of GI bleeding; NSAIDs can potentiate anticoagulant effect.
- Other NSAIDs or high‑dose salicylates
- Additive GI and renal toxicity; generally avoid combination.
- Other paracetamol‑containing products
- Increases total daily paracetamol exposure and risk of liver injury.
- Hepatotoxic drugs (e.g., isoniazid, rifampicin, certain antiepileptics, high‑dose methotrexate)
- Additive hepatotoxic risk; careful monitoring or alternative analgesic recommended.
- Diuretics, ACE inhibitors, ARBs
- NSAID‑related reduction in renal perfusion may blunt antihypertensive effect and increase risk of renal dysfunction.
- Alcohol
- Synergistic hepatotoxicity with both nimesulide and paracetamol.
Pharmacovigilance‑oriented prescribing should include medication reconciliation and documentation of all OTC and herbal agents that may affect liver enzymes or bleeding risk.
Regulatory considerations
Global snapshot
- Nimesulide has faced extensive regulatory scrutiny due to hepatotoxicity signals, with suspension or withdrawal of oral formulations in several countries.
- EMA’s comprehensive review concluded in 2012 that continued use could be justified only with strict limits: maximum daily dose 200 mg, maximum duration 15 days, and second‑line use for acute pain and primary dysmenorrhoea.
India‑specific context
- In India, nimesulide continues to be marketed as single‑ingredient and in FDCs with paracetamol and other agents for acute pain and fever.
- Regulatory and expert bodies (e.g., ICMR, DTAB, CDSCO) have periodically reviewed safety; recent recommendations include:
- Avoiding oral formulations above 100 mg immediate‑release per dose.
- Discouraging use in pregnant or lactating women, those planning pregnancy, and patients with hepatic or renal impairment.
- Seeking additional data on use in children, adolescents, and older adults to further define restrictions.
For pharma manufacturers and marketers, this means:
- Strong emphasis on short‑term use, precise labeling of contraindications, and inclusion of clear liver‑warning boxes.
- Robust pharmacovigilance, ADR reporting, and periodic safety update reports.
- Conservative promotional claims, avoiding any suggestion of chronic use or “safe long‑term painkiller.”
Latest research and controversies around nimesulide
- Systematic reviews continue to support a real but infrequent risk of serious hepatotoxicity, with higher relative risk compared to some other NSAIDs.
- Recent Indian policy discussions have reignited debate about risk–benefit in a market where cheaper, safer alternatives (e.g., ibuprofen, naproxen, paracetamol alone) are widely available.
- Many authors argue that, when used at recommended doses for very short durations in carefully selected adults, nimesulide may still have a role, especially where rapid onset and good GI tolerability are valued.
- However, patient‑level risk factors, inappropriate OTC sale, and polypharmacy increase real‑world risk, which regulators and industry must actively address.
Comparison with other analgesic–antipyretic options
Positioning vs common combinations
| Feature / Aspect | Nimesulide + Paracetamol Tablets | Paracetamol Alone | Ibuprofen + Paracetamol | Diclofenac Tablets |
|---|---|---|---|---|
| Main actions | NSAID (relatively COX‑2 selective) + central analgesic/antipyretic | Central analgesic/antipyretic | Non‑selective NSAID + central analgesic/antipyretic | Non‑selective NSAID, strong peripheral analgesic |
| Typical Indian acute pain use | Dental, musculoskeletal, dysmenorrhoea, febrile pain when monotherapy inadequate | First‑line for fever, headache, mild to moderate pain | Short‑term moderate pain and fever in adults and older children | Acute musculoskeletal pain, postoperative pain, arthritis flares |
| Hepatotoxicity profile | Significant concern; documented cases of severe liver injury and regulatory restrictions | Dose‑dependent; mainly in overdose, alcohol misuse, liver disease | Added paracetamol risk; ibuprofen generally lower hepatotoxicity than nimesulide | Primarily GI and CV risk; liver injury less prominent but reported |
| GI safety | Often better GI tolerance vs some older NSAIDs but still ulcer/bleed risk | Very low GI ulcer risk at usual doses | NSAID‑related ulcer risk, mitigated at OTC doses | Higher GI ulcer/bleed risk; often needs gastroprotection in high‑risk adults |
| Regulatory stance globally | Restricted or withdrawn in many countries; strict EU limits on dose/duration | Widely accepted first‑line worldwide | Widely used; some countries approve FDCs | Widely used but with growing CV and GI safety warnings |
For a pharma entrepreneur, the commercial opportunity lies in differentiated positioning, strict risk‑mitigation messaging, and possible transition strategies toward safer combinations while responding to evolving Indian regulatory guidance.
Practical considerations for pharma manufacturers and marketers
- Ensure DCGI‑compliant labeling that highlights: dose limitations, maximum duration, liver warnings, and contraindications.
- Implement robust field‑force training so that MRs promote short‑term, indication‑specific use and avoid off‑label chronic pain positioning.
- Maintain active pharmacovigilance, capturing ADRs, especially liver and GI events, and reporting them as per Indian PV regulations.
- Consider parallel development of safer analgesic combinations to hedge against possible future tightening or withdrawal of nimesulide FDCs.
FAQ – Nimesulide and Paracetamol Tablets
What are Nimesulide and Paracetamol Tablets used for?
They are used for short‑term relief of pain and fever in conditions such as headache, toothache, muscle and joint pain, and menstrual pain when a single drug is not sufficient.
Is nimesulide banned in India?
Nimesulide is not fully banned in India but remains under regulatory scrutiny; some uses and strengths are restricted, and expert committees have recommended caution, especially in children, pregnant women, and patients with liver problems.
How long can a patient take Nimesulide and Paracetamol Tablets?
Regulatory reviews support use only for short periods, typically a few days and not beyond 15 days, with many clinicians preferring ≤3–5 days in routine acute pain.
Can these tablets be taken with alcohol?
No. Alcohol increases the chance of liver damage from both nimesulide and paracetamol and should be strictly avoided during treatment.
Are Nimesulide and Paracetamol Tablets safe in children?
Many guidelines advise against use in young children; typical Indian labels restrict use below certain age or weight thresholds, and regulators have asked for more safety data in paediatric populations.
Can pregnant or breastfeeding women take this combination?
Use is generally discouraged in pregnancy and lactation due to limited controlled data and potential fetal/neonatal risk; safer alternatives are usually preferred.
What signs of liver damage should be monitored?
Yellowing of skin or eyes, dark urine, persistent nausea, severe fatigue, and upper‑right abdominal pain are warning signs requiring immediate medical review and discontinuation.
References:
- Nimesulide – Wikipedia. Available from: https://en.wikipedia.org/wiki/Nimesulide.
- Sarganas G, et al. Nimesulide‑induced hepatotoxicity: a systematic review and meta‑analysis. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC6345488/.
- European Medicines Agency. Nimesulide – referral (liver safety and benefit–risk review). Available from: https://www.ema.europa.eu/en/medicines/human/referrals/nimesulide.
- European Medicines Agency. Nimesulide – referral (final opinion and restrictions). Available from: https://www.ema.europa.eu/en/medicines/human/referrals/nimesulide-1.
- DrugBank. Nimesulide: Uses, Interactions, Mechanism of Action. Available from: https://go.drugbank.com/drugs/DB04743.
- Rainsford KD. Clinical pharmacokinetics of nimesulide. Available from: https://pubmed.ncbi.nlm.nih.gov/9812177/.
- Review: Nimesulide – some pharmaceutical and pharmacological aspects. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1211/0022357001774255.
- JAPI. Nimesulide: critical appraisal of safety and efficacy in acute pain. Available from: https://japi.org/article/japi-73-3-e22.
- IRJMETS. Global ban on nimesulide. Available from: https://www.irjmets.com/upload_newfiles/irjmets71100114191/paper_file/irjmets71100114191.pdf.
- Medical Dialogues. DTAB, CDSCO recommend stringent restrictions on nimesulide. Available from: https://medicaldialogues.in/news/industry/pharma/not-a-nice-time-for-dr-reddys-dtab-cdsco-recommends-stringent-restrictions-on-nimesulide.
- Apollo Pharmacy. Nimesulide + Paracetamol: Uses, Side Effects and Medicines. Available from: https://www.apollopharmacy.in/salt/Nimesulide+paracetamol.
- 1mg. Nimesulide + Paracetamol: uses, side effects and medicines. Available from: https://www.1mg.com/generics/nimesulide-paracetamol-402515.
- ZeeLab Pharmacy. Nimesulide + Paracetamol – uses, benefits, side effects & price. Available from: https://zeelabpharmacy.com/generic-salt/nimesulide-paracetamol.
- Care Hospitals. Nimesulide: uses, side effects, dosage, precautions & more. Available from: https://www.carehospitals.com/medicine-detail/nimesulide.
- Waylone Healthcare. Nimesulide + Paracetamol Tablets product page. Available from: https://waylonehealthcare.in/product/nimesulide-100-mg-paracetamol-325-mg/.
- Cohiba Pharmaceuticals. Nimesulide & Paracetamol Tablets product page. Available from: https://www.cohibapharma.com/product/twignim-p/.
Reviewed by;

Dr. Yogesh Chaudhary (B. Pharma)
Senior Pharmacist at S.N. Medical College, Agra-(UP)

