This comprehensive article provides an in-depth analysis of ZEGFROVY™ (sunvozertinib), a recently FDA-approved kinase inhibitor, drawing insights from its official prescribing information and briefing documents. Tailored for healthcare professionals and informed pharmaceutical stakeholders in both India and the United States, this piece delves into the drug’s therapeutic applications, mechanism, clinical performance, safety profile, and regulatory journey, offering critical perspectives on its integration into evolving oncology practices.
Overview of the Drug
Non-small cell lung cancer (NSCLC) remains a formidable challenge in oncology, accounting for the vast majority of lung cancer diagnoses. Within this complex landscape, specific genetic alterations drive tumor growth and progression, making targeted therapies a cornerstone of modern treatment. Among these, epidermal growth factor receptor (EGFR) mutations are particularly prevalent, with a distinct subset being EGFR exon 20 insertion mutations. Historically, these insertions have been notoriously difficult to treat, often exhibiting resistance to first and second-generation EGFR tyrosine kinase inhibitors (TKIs), leaving patients with limited therapeutic options.
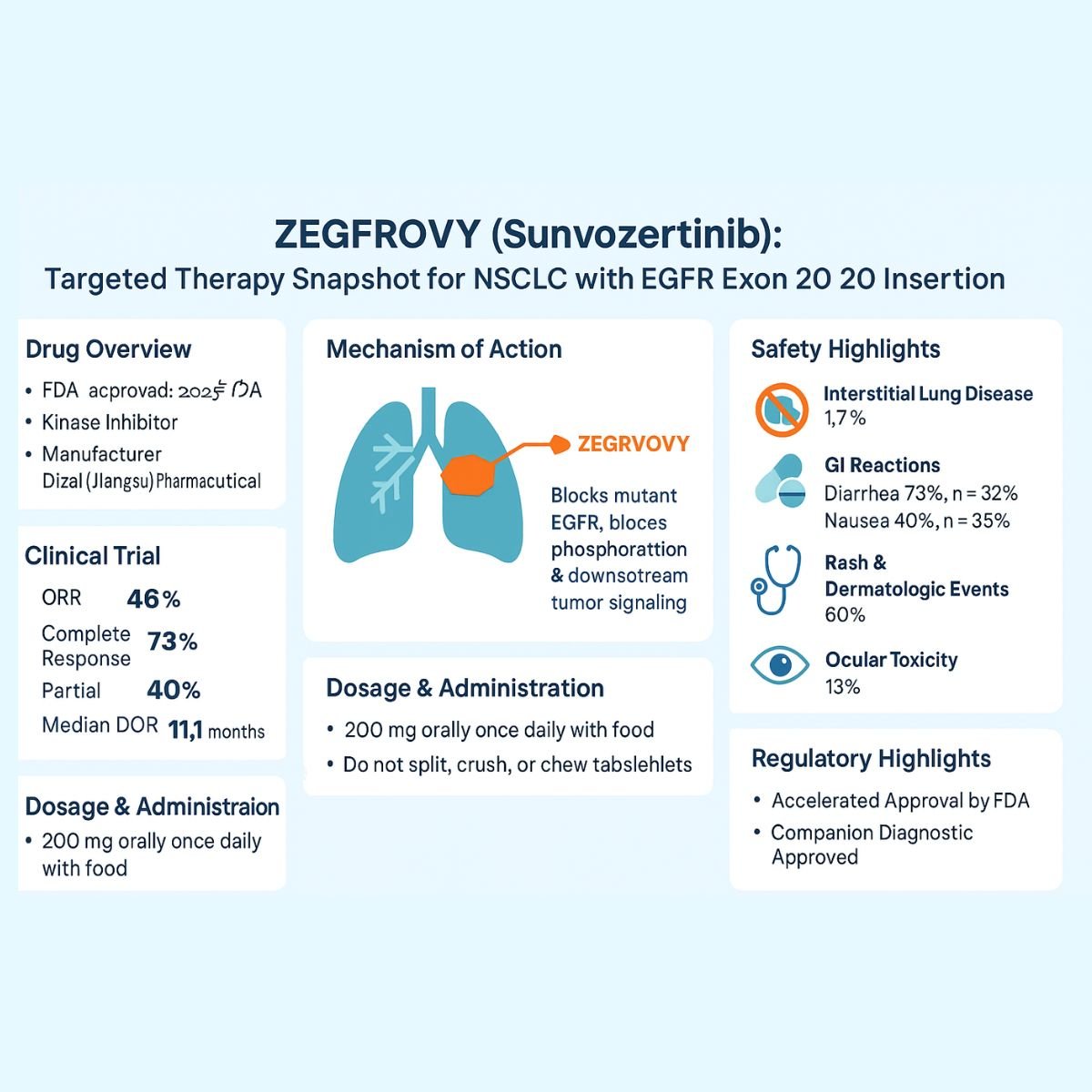
In a significant advancement for this patient population, ZEGFROVY™ (sunvozertinib) has received accelerated approval from the U.S. Food and Drug Administration (FDA). Sunvozertinib represents a novel oral kinase inhibitor specifically designed to address the unmet medical needs of adult patients with locally advanced or metastatic NSCLC harboring these challenging EGFR exon 20 insertion mutations. This approval marks a pivotal moment, offering a new targeted approach for patients whose disease has progressed following platinum-based chemotherapy, a standard first-line treatment. The introduction of ZEGFROVY underscores the continuous evolution of precision medicine in oncology, aiming to provide more effective and tailored therapies for genetically defined patient subsets.
ZEGFROVY™
Drug Overview
Key Highlights
Indication
For adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with EGFR exon 20 insertion mutations, whose disease has progressed on or after platinum-based chemotherapy.
Developer
Dizal (Jiangsu) Pharmaceutical Co., Ltd.
Approval Status
FDA Accelerated Approval – July 2, 2025
Target Population
Adults with platinum-refractory NSCLC
The Challenge
EGFR exon 20 insertion mutations have historically been resistant to traditional EGFR tyrosine kinase inhibitors (TKIs).
Breakthrough
Sunvozertinib is a novel, oral EGFR TKI with high selectivity for exon 20 insertion mutations over wild-type EGFR.
Pivotal Trial
The WU-KONG6 trial demonstrated significant and durable responses in heavily pretreated patients.
FDA Approval
Accelerated approval was granted, marking a new standard of care for this patient population.
Drug Composition & Formulation
Molecular Structure
Molecular Weight: 577.7 g/mol
Tablet Strengths
Supplied as 100 mg and 150 mg film-coated tablets.
Appearance
Specific appearance details (color, debossing) are found in the full prescribing information.
Mechanism of Action
Precision Targeting
Sunvozertinib is a kinase inhibitor that selectively and irreversibly binds to EGFR proteins with exon 20 insertion mutations, leading to the inhibition of tumor cell growth.
| Action | Biological Impact | Clinical Significance |
|---|---|---|
| Selective Inhibition | Inhibits mutant EGFR at lower concentrations than wild-type (WT) EGFR. | Reduces off-target side effects commonly seen with less selective TKIs. |
| Irreversible Binding | Forms a covalent bond with the cysteine residue in the ATP-binding site of EGFR. | Provides sustained suppression of EGFR signaling pathways. |
| Anti-Tumor Activity | Induces apoptosis (cell death) and inhibits proliferation of cancer cells harboring EGFR exon20ins mutations. | Leads to tumor shrinkage and durable clinical responses. |
Clinical Efficacy Data (WU-KONG6 Trial)
Pivotal Trial (NCT05559733)
An open-label, single-arm multicenter study in 97 adult patients with locally advanced or metastatic NSCLC with EGFR Exon20ins mutations who had progressed on or after platinum-based chemotherapy.
Duration of Response
59% of patients achieved a Duration of Response ≥6 months
Safety Profile & Adverse Events
Warnings & Precautions
- Interstitial Lung Disease (ILD)/Pneumonitis: Can be fatal. Monitor for new or worsening pulmonary symptoms.
- Diarrhea: Can be severe. Initiate antidiarrheal treatment, increase fluids, and modify dose as needed.
- QTc Interval Prolongation: Monitor ECGs and electrolytes, especially in at-risk patients.
- Cardiomyopathy: Can lead to cardiac failure. Assess LVEF before and during treatment.
- Ocular Toxicity: Keratitis can occur. Promptly refer patients with eye problems.
Most Common Adverse Reactions (≥20%)
The most frequent adverse reactions were diarrhea, rash, paronychia, stomatitis, decreased appetite, and anemia.
Drug Interactions
Critical CYP3A-Mediated Interactions
Sunvozertinib is primarily metabolized by the CYP3A enzyme. Co-administration with drugs that affect CYP3A can alter sunvozertinib exposure.
| Concomitant Drug Type | Effect on ZEGFROVY | Recommendation | Examples |
|---|---|---|---|
| Strong/Moderate CYP3A Inhibitors | ↑ Increases exposure | Avoid co-administration. If unavoidable, reduce ZEGFROVY dose. | Ketoconazole, itraconazole, clarithromycin, ritonavir |
| Strong/Moderate CYP3A Inducers | ↓ Decreases exposure | Avoid co-administration as it may reduce efficacy. | Rifampin, carbamazepine, St. John’s Wort, phenytoin |
| Drugs that Prolong QTc | ↑ Risk of QTc prolongation | Avoid co-administration. If unavoidable, monitor ECGs more frequently. | Anti-arrhythmics, certain antipsychotics, macrolides |
Clinical Practice Guidelines
Dosing & Administration
Recommended Dose
300 mg orally once daily.
Administration
Take with or without food, at approximately the same time each day. Swallow tablets whole.
Monitoring Requirements
| Parameter | Frequency | Purpose |
|---|---|---|
| Pulmonary Symptoms | At each visit | Monitor for ILD/Pneumonitis |
| ECG & Electrolytes | Baseline, then periodically | Monitor for QTc prolongation |
| LVEF | Baseline, then periodically | Monitor for Cardiomyopathy |
Frequently Asked Questions
ZEGFROVY (sunvozertinib) is specifically designed to target EGFR exon 20 insertion mutations, which are historically resistant to first and second-generation EGFR inhibitors. Its high selectivity for the mutated protein over the normal (wild-type) EGFR leads to strong efficacy with a manageable safety profile.
Diarrhea is a very common side effect. Patients should be advised to start standard antidiarrheal therapy (like loperamide) at the first sign of loose stools, maintain adequate hydration, and follow a BRAT diet. For severe or persistent diarrhea, the dose of ZEGFROVY may need to be interrupted or reduced by their doctor.
Yes. Patients must have confirmation of an EGFR exon 20 insertion mutation using an FDA-approved test before starting ZEGFROVY. This ensures the treatment is targeted to the patients most likely to benefit.
Therapeutic Uses: Precision in NSCLC Treatment
ZEGFROVY’s indication is highly specific, reflecting the principles of precision oncology. It is approved for the treatment of adult patients diagnosed with:
- Locally advanced or metastatic non-small cell lung cancer (NSCLC): This specifies the stage of the disease for which ZEGFROVY is applicable, targeting patients with advanced disease that has either spread to nearby tissues or metastasized to distant sites.
- Epidermal growth factor receptor (EGFR) exon 20 insertion mutations: This is the critical genetic biomarker for patient selection. The presence of these specific mutations, as detected by an FDA-approved test, is mandatory for ZEGFROVY eligibility. This highlights the indispensable role of comprehensive genomic profiling in contemporary NSCLC management.
- Disease progression on or after platinum-based chemotherapy: This criterion defines the treatment line for ZEGFROVY. It is intended for patients who have already undergone standard platinum-based chemotherapy and whose disease has either continued to progress during or after this treatment. This positions ZEGFROVY as a second-line or later therapy, addressing a population with limited remaining options.
The approval of ZEGFROVY under the accelerated approval pathway is particularly noteworthy. This regulatory mechanism allows for earlier access to promising drugs for serious conditions based on surrogate endpoints, such as overall response rate (ORR) and duration of response (DOR), which are reasonably likely to predict clinical benefit. While this provides expedited access, it also means that continued approval for this indication is contingent upon verification and description of clinical benefit in confirmatory trial(s). This emphasizes the ongoing commitment to rigorous evidence generation even after initial market entry.
Also Read: EMRELIS For NSCLC (Telisotuzumab Vedotin): Targeted Hope or Cautious Bet?
Mechanism of Action
Sunvozertinib’s mechanism of action is central to its therapeutic efficacy in EGFR exon 20 insertion mutation-positive NSCLC. As a kinase inhibitor, sunvozertinib specifically targets the epidermal growth factor receptor (EGFR), a transmembrane protein that plays a crucial role in cell growth, proliferation, and survival. In many cancers, including NSCLC, EGFR can become aberrantly activated due to mutations, leading to uncontrolled cell division and tumor progression.
- Targeting EGFR Exon 20 Insertion Mutations: Unlike common EGFR mutations (e.g., exon 19 deletions or L858R point mutations) that are sensitive to earlier generation EGFR TKIs, exon 20 insertion mutations are structurally distinct and typically confer resistance to these drugs. Sunvozertinib is engineered to overcome this resistance. It binds to and inhibits EGFR exon 20 insertion mutations, demonstrating a unique binding profile that allows it to effectively block the aberrant signaling pathways driven by these specific alterations.
- Differential Inhibition Profile: In vitro cell models have provided critical insights into sunvozertinib’s selectivity. These studies showed that sunvozertinib inhibited EGFR phosphorylation in cells expressing various EGFR exon 20 insertion mutation variants at approximately 2- to 10-fold lower concentrations compared to its inhibition of wild-type EGFR signaling. This differential inhibition is crucial, as it suggests a degree of selectivity for the mutated EGFR, potentially leading to a more favorable therapeutic index by minimizing off-target effects on normal cells expressing wild-type EGFR.
- Anti-tumor Activity in Xenograft Models: Beyond in vitro observations, sunvozertinib has demonstrated robust anti-tumor activity in xenograft models of NSCLC with EGFR exon 20 insertion mutations. These in vivo studies, conducted in animal models where human tumors are implanted, provide compelling evidence of the drug’s ability to reduce tumor size and inhibit tumor growth, further supporting its clinical potential. This comprehensive understanding of its mechanism underscores sunvozertinib’s role as a precisely targeted therapy, addressing a specific molecular vulnerability in NSCLC.
Dosage & Administration:
The successful integration of any new therapeutic agent into clinical practice hinges on clear and practical dosage and administration guidelines. For ZEGFROVY, adherence to these recommendations is paramount for optimizing efficacy and managing potential toxicities.
- Recommended Dosage: The standard recommended dosage of ZEGFROVY is 200 mg orally once daily. This consistent daily regimen aims to maintain therapeutic drug concentrations and maximize anti-tumor activity.
- Administration with Food: A critical instruction is that ZEGFROVY must be taken with food. This is not merely a suggestion but a requirement, as food intake can significantly influence the drug’s absorption and bioavailability, potentially reducing gastrointestinal adverse reactions. Healthcare professionals should emphasize this point strongly to patients to ensure consistent drug exposure and minimize GI side effects.
- Tablet Integrity: Patients are instructed to swallow ZEGFROVY tablets whole. The tablets should not be split, crushed, chewed, or dissolved. This is crucial to preserve the integrity of the tablet formulation, which is designed to ensure proper drug release and absorption. Altering the tablet can lead to unpredictable pharmacokinetics, potentially affecting efficacy or increasing toxicity.
- Consistent Dosing Time: Patients are advised to take ZEGFROVY at the same time each day. Establishing a routine helps maintain steady-state drug levels and improves patient adherence, which is vital for long-term treatment success in chronic conditions like cancer.
- Missed Dose Management:
- Within 12 hours: If a dose of ZEGFROVY is missed within 12 hours of the regularly scheduled time, the patient should take the missed dose as soon as they remember.
- More than 12 hours: If more than 12 hours have passed since the scheduled dose, the patient should skip the missed dose entirely and take the next dose at their regularly scheduled time. This prevents accidental double dosing, which could increase the risk of adverse events.
- Vomiting After Dose: In the event that a patient vomits after taking a dose of ZEGFROVY, they should not take an additional dose. Instead, they should resume their regular dosing schedule with the next planned dose. This directive is in place to avoid over-dosing, considering that some of the drug may have been absorbed prior to vomiting.
- Dosage Forms and Strengths: ZEGFROVY is available in two strengths: 150 mg and 200 mg. Both are presented as yellow, biconvex film-coated tablets, debossed with their respective strengths and the Dizal company logo for easy identification.
- Dosage Modifications for Adverse Reactions: Managing adverse reactions is a key aspect of TKI therapy. The dose of ZEGFROVY may be reduced to 150 mg orally once daily for the management of specific adverse reactions. If a patient is unable to tolerate the 150 mg dose, permanent discontinuation of ZEGFROVY is advised. Detailed guidelines for dosage modifications related to Interstitial Lung Disease (ILD)/Pneumonitis, gastrointestinal toxicities, dermatologic reactions, and ocular toxicity are provided in the prescribing information, emphasizing a proactive and individualized approach to patient management. Healthcare professionals must be familiar with these guidelines to ensure patient safety and optimize treatment continuity.
Clinical Trials Summary:
The accelerated approval of ZEGFROVY is primarily underpinned by the efficacy data derived from the WU-KONG1B (NCT03974022) clinical trial. This multinational, open-label, dose randomization study played a pivotal role in demonstrating the drug’s clinical benefit in a challenging patient population.
- Study Design and Population: The WU-KONG1B study enrolled 85 adult patients with locally advanced or metastatic NSCLC who harbored confirmed EGFR exon 20 insertion mutations. Crucially, all enrolled patients had experienced disease progression on or after platinum-based chemotherapy, representing a heavily pre-treated cohort with limited therapeutic alternatives. Patients received ZEGFROVY at the recommended dose of 200 mg orally once daily. The open-label design allowed for direct observation of treatment effects, while the multinational nature provided a broader representation of the patient population.
- Key Efficacy Outcomes: The primary efficacy outcome measure was the Overall Response Rate (ORR), assessed by a blinded independent review committee (BIRC) using Response Evaluation Criteria in Solid Tumors (RECIST v1.1). ORR is a critical surrogate endpoint in oncology, indicating the proportion of patients whose tumors shrink or disappear in response to treatment.
- Overall Response Rate (ORR): ZEGFROVY demonstrated a compelling ORR of 46% (95% CI: 35, 57). This means nearly half of the patients in the study experienced a significant reduction in tumor size. The breakdown included 6% complete responses (CR), where all target lesions disappeared, and 40% partial responses (PR), indicating a substantial reduction in tumor size. This response rate is particularly encouraging given the historical difficulty in treating EGFR exon 20 insertion mutations.
- Duration of Response (DOR): An equally important efficacy outcome was the Duration of Response (DOR), also evaluated by BIRC. DOR measures how long the tumor response is maintained. The median DOR observed was 11.1 months (95% CI: 8.2, not evaluable). Furthermore, a significant proportion of patients, 72%, had a DOR of 6 months or longer. A sustained response is crucial for improving patient outcomes and quality of life in advanced cancer settings.
- Implications of Accelerated Approval: The accelerated approval pathway, utilized for ZEGFROVY, reflects the urgent unmet need for effective therapies in this specific NSCLC subtype. While the ORR and DOR data are robust and clinically meaningful, the “accelerated” nature of the approval means that continued market authorization is contingent upon the verification of clinical benefit in ongoing or future confirmatory trials. This mechanism allows patients to access promising treatments sooner while further data are collected to solidify long-term efficacy and overall survival benefits.
- Companion Diagnostic: The approval of ZEGFROVY was accompanied by the FDA approval of a companion diagnostic device, the Oncomine™ Dx Express Test (Life Technologies Corporation). This emphasizes the critical role of precise patient selection. Healthcare professionals must ensure that patients are tested for EGFR exon 20 insertion mutations using an FDA-approved test to confirm eligibility for ZEGFROVY treatment. This approach ensures that the right therapy is delivered to the right patient, maximizing the likelihood of a positive outcome.
Adverse Events & Safety Profile:
Like all potent oncology agents, ZEGFROVY is associated with a distinct safety profile that requires careful monitoring and proactive management by healthcare professionals. The prescribing information highlights several serious potential adverse reactions (ARs) that warrant particular attention.
- Interstitial Lung Disease (ILD)/Pneumonitis:
- Description: This is a severe and potentially life-threatening complication, occurring in 1.7% of patients in the safety population. The median time to onset was 61 days.
- Management: Healthcare professionals must monitor patients for new or worsening pulmonary symptoms (e.g., dyspnea, cough, fever). If ILD/pneumonitis is suspected, ZEGFROVY must be immediately withheld. If confirmed, permanent discontinuation is required. This necessitates a high index of suspicion and prompt intervention.
- Gastrointestinal Adverse Reactions:
- Description: Severe gastrointestinal ARs, including diarrhea (73% overall, 2.5% Grade 3), nausea, and vomiting (43% overall, 3.3% Grade 3), are common.
- Management: Administering ZEGFROVY with food is recommended to mitigate these effects. Proactive supportive care, including anti-diarrheals (e.g., loperamide), anti-emetics, and fluid replacement, is essential. Dose withholding, reduction, or permanent discontinuation may be necessary based on severity. Patient education on managing these symptoms at home is crucial.
- Dermatologic Adverse Reactions:
- Description: Rash, including acneiform dermatitis and pruritus, occurred in 68% of patients, with 7% experiencing Grade 3 rash.
- Management: Patients should be advised to use alcohol-free emollient creams and avoid irritating skin products (e.g., those containing retinol, retinoic acid, benzoyl peroxides). Dose modifications (withholding, reduction, or permanent discontinuation) are guided by the severity of the reaction. Early intervention with topical or systemic treatments can prevent progression to severe forms.
- Ocular Toxicity:
- Description: Ocular toxicities, including keratitis, dry eye symptoms, blurred vision, and visual impairment, were observed in 13% of patients (0.8% keratitis).
- Management: Patients presenting with new or worsening eye symptoms should be promptly referred to an ophthalmologist. Discontinuation of contact lenses is advised until symptoms are evaluated. Dose modifications are based on severity, with permanent discontinuation if ulcerative keratitis is confirmed. Regular ophthalmic monitoring may be considered for high-risk patients.
- Embryo-Fetal Toxicity:
- Description: Based on animal studies and its mechanism of action, ZEGFROVY can cause fetal harm.
- Management: This is a critical warning. Females of reproductive potential must be advised of the potential risk and use effective non-hormonal contraception during treatment and for 2 weeks after the last dose, as hormonal contraceptives may be rendered ineffective. Pregnancy status must be verified before initiation. Male patients with female partners of reproductive potential also need to use effective contraception during treatment and for 2 weeks after the last dose.
- Infertility: Animal studies suggest ZEGFROVY may impair fertility in females (reversibility not assessed) and males (reversible effects). This should be discussed with patients of reproductive age.
- Most Common Adverse Reactions (≥20%): Beyond the serious warnings, common adverse events include diarrhea, rash, decreased appetite, stomatitis, fatigue, nausea, paronychia, vomiting, constipation, musculoskeletal pain, pruritus, dry skin, urinary tract infection, abdominal pain, and decreased weight. While generally manageable, these can impact patient quality of life and adherence.
- Serious and Fatal Adverse Reactions: Serious ARs occurred in 41% of patients, with pneumonia (9%), dyspnea (4.4%), pancreatitis, device-related infection, and rash (2.2% each) being most common. Fatal ARs occurred in 2.2% of patients (thrombosis and COVID-19 infection, 1.1% each). Permanent discontinuation due to ARs occurred in 8% of patients (pneumonia and rash each 2.2%).
- Laboratory Abnormalities: Common Grade 3 or 4 laboratory abnormalities (≥2%) included decreased lymphocytes, increased lipase, decreased hemoglobin, increased amylase, increased creatine kinase, and decreased neutrophils. Regular laboratory monitoring is crucial.
Pharmacodynamics & Pharmacokinetics
A thorough understanding of ZEGFROVY’s pharmacodynamics (PD) and pharmacokinetics (PK) is essential for healthcare professionals to predict its behavior in the body, optimize dosing, and anticipate potential drug interactions.
- Pharmacokinetics (PK):
- Absorption: Sunvozertinib reaches its median maximum plasma concentration (tmax) approximately 6 hours post-dose. Importantly, food intake does not significantly affect its overall exposure (AUC) or peak concentration (Cmax), reinforcing the “with food” administration instruction primarily for GI tolerability rather than absorption.
- Distribution: The apparent (oral) volume of distribution is substantial at 2,116 L, indicating wide distribution into tissues. In vitro plasma protein binding ranges from 89% to 94%.
- Elimination: Sunvozertinib has a relatively long elimination half-life of 50 hours, supporting its once-daily dosing regimen. The apparent (oral) clearance is 29 L/h. The majority of the dose (79%) is recovered in feces, with a smaller proportion (10%) excreted in urine.
- Metabolism: Sunvozertinib is primarily metabolized by the cytochrome P450 3A (CYP3A) enzyme system. It forms an active demethylated metabolite, DZ0753, which contributes approximately 10% to the parent drug’s overall exposure (AUC).
- Specific Populations: No clinically significant differences in sunvozertinib pharmacokinetics were observed based on age (19 to 96 years), sex, race (Asian 62%, White 28%, Black or African American 8%), body weight, smoking status, or mild to moderate renal and hepatic impairment. The effects of severe renal or hepatic impairment have not been studied.
- Pharmacodynamics (PD):
- Exposure-Response Relationships: Clinical studies did not observe clinically significant exposure-response relationships for overall response rate (ORR) across the tested dose range (200 mg to 300 mg), suggesting that the 200 mg dose is within the effective therapeutic window.
- Cardiac Electrophysiology: At 1.5 times the approved recommended dose, no clinically significant QTc interval prolongation was observed, providing reassurance regarding cardiac safety.
- Drug Interactions: Given its metabolism by CYP3A and its effects on other transporters, sunvozertinib has several important drug interactions:
- Strong CYP3A Inhibitors: Concomitant use should be avoided. If unavoidable, the ZEGFROVY dose should be reduced (e.g., from 200 mg to 150 mg), and patients should be monitored for increased adverse reactions, as strong CYP3A inhibitors (e.g., itraconazole) can significantly increase sunvozertinib exposure.
- Strong and Moderate CYP3A Inducers: Concomitant use should also be avoided. If unavoidable, the ZEGFROVY dose may need to be increased (e.g., from 200 mg to 400 mg), as these inducers (e.g., carbamazepine, efavirenz) can decrease sunvozertinib exposure, potentially reducing its effectiveness.
- Hormonal Contraceptives (CYP3A Substrates): Concomitant use is to be avoided. ZEGFROVY can induce CYP3A4, potentially rendering hormonal contraceptives ineffective. Females of reproductive potential should use effective non-hormonal contraception.
- P-gp or BCRP Substrates: ZEGFROVY inhibits P-gp and BCRP. Coadministration with substrates of these transporters (e.g., digoxin, rosuvastatin) may increase their exposure, necessitating monitoring for increased adverse reactions.
Regulatory Path
The journey of ZEGFROVY to market exemplifies a strategic regulatory approach designed to bring innovative therapies to patients with high unmet medical needs as quickly as possible.
- Accelerated Approval Pathway: ZEGFROVY received accelerated approval from the FDA. This pathway is a crucial mechanism for expediting the availability of drugs for serious conditions that demonstrate meaningful therapeutic advantage over existing treatments. It relies on surrogate endpoints (like ORR and DOR in this case) that are reasonably likely to predict clinical benefit. This allows patients to gain access to potentially life-saving treatments years earlier than through traditional approval pathways. For ZEGFROVY, the compelling response rates and duration of response observed in the WU-KONG1B trial were sufficient to support this expedited approval, highlighting the significant impact it could have on patients with EGFR exon 20 insertion mutation-positive NSCLC.
- Post-Marketing Requirements (Confirmatory Trials): A key aspect of accelerated approval is the requirement for post-marketing confirmatory trials. These trials are designed to verify and describe the clinical benefit, often focusing on hard clinical endpoints like overall survival or progression-free survival. For ZEGFROVY, continued approval for this indication is contingent upon the successful completion and positive results of such confirmatory studies. This ensures that while patients gain early access, the full long-term benefits and safety profile are rigorously evaluated.
- Companion Diagnostic Approval: The approval of ZEGFROVY was intrinsically linked to the simultaneous FDA approval of a companion diagnostic device, the Oncomine™ Dx Express Test. This co-development and co-approval underscore the paradigm of precision medicine, where a specific diagnostic test is essential to identify the patient population most likely to benefit from the targeted therapy. This ensures appropriate patient selection, maximizing the drug’s efficacy and minimizing exposure in non-responders. The availability of an FDA-approved test provides a standardized and validated method for identifying EGFR exon 20 insertion mutations in tumor tissue.
Manufacturer & Approval Milestones
The development and approval of ZEGFROVY represent a significant milestone for its manufacturer and for the global oncology community.
- Manufacturer: ZEGFROVY is manufactured for Dizal (Jiangsu) Pharmaceutical Co., Ltd., based in Shanghai, China. This highlights the growing contribution of pharmaceutical companies from emerging markets to global drug discovery and development, particularly in highly specialized therapeutic areas like targeted oncology. Dizal’s focus on innovative therapies for unmet medical needs is exemplified by this approval.
- Initial U.S. Approval: The initial U.S. approval date for ZEGFROVY was 2025. This date marks its entry into one of the world’s largest and most regulated pharmaceutical markets, signifying a rigorous review process and validation of its clinical utility.
- Trademark: ZEGFROVY™ is a registered trademark of Dizal (Jiangsu) Pharmaceutical Co., Ltd., reinforcing the proprietary nature of this novel therapeutic agent. The successful navigation of the FDA approval process by Dizal underscores its capabilities in drug development, clinical trials, and regulatory affairs on an international scale. This approval not only provides a new treatment option for patients but also elevates Dizal’s standing as a key player in the global oncology landscape.
The Evolving Landscape of NSCLC Treatment
The approval of ZEGFROVY is not an isolated event but rather a testament to the ongoing paradigm shift in NSCLC treatment from broad-spectrum chemotherapy to highly precise, mutation-driven targeted therapies. For decades, NSCLC management relied heavily on cytotoxic chemotherapy, often associated with significant systemic toxicities and limited long-term efficacy in advanced stages. The discovery of oncogenic drivers like EGFR mutations revolutionized this approach, ushering in the era of precision oncology.
However, even within EGFR-mutated NSCLC, the exon 20 insertion mutations presented a unique challenge. Their distinct molecular structure often rendered them resistant to the first and second-generation EGFR TKIs that proved highly effective for other common EGFR mutations. This left a significant subset of patients with limited effective targeted options, often reverting to less effective and more toxic conventional chemotherapy or investigational agents. ZEGFROVY’s targeted action against these specific insertions fills a critical therapeutic gap, offering a much-needed, effective, and well-tolerated (relative to chemotherapy) treatment option for this previously underserved patient population. This continuous refinement of targeted therapies, moving from broad classes to highly specific molecular subsets, is the future of cancer care, promising improved outcomes and reduced treatment burden.
Implications for Clinical Practice
The approval of ZEGFROVY carries significant implications for clinical practice in both the United States and India, albeit with nuanced considerations due to differences in healthcare systems, regulatory frameworks, access to diagnostics, and patient demographics.
H3: Implications in the United States
- Enhanced Treatment Options: In the US, ZEGFROVY provides a crucial new second-line (or later) targeted therapy for patients with EGFR exon 20 insertion mutation-positive NSCLC who have progressed on platinum-based chemotherapy. This expands the therapeutic armamentarium for a patient population that previously had limited effective options.
- Importance of Comprehensive Genomic Profiling (CGP): The requirement for an FDA-approved companion diagnostic (Oncomine™ Dx Express Test) reinforces the necessity of comprehensive genomic profiling for all NSCLC patients at diagnosis or progression. Oncologists will need to ensure that their patients undergo appropriate testing to identify these specific mutations.
- Integration into Guidelines: Over time, ZEGFROVY is expected to be integrated into national clinical practice guidelines (e.g., NCCN guidelines), providing standardized recommendations for its use.
- Access and Reimbursement: As a new targeted therapy, access and reimbursement will be key considerations. Healthcare providers and patients will navigate insurance coverage and financial assistance programs.
- Adverse Event Management: US healthcare professionals are generally well-versed in managing TKI-associated toxicities. However, specific education on ZEGFROVY’s unique safety profile, especially regarding ILD/pneumonitis and ocular toxicity, will be important.
H3: Implications in India
- Addressing a High Burden of NSCLC: India faces a rapidly increasing burden of NSCLC. The availability of a targeted therapy for a specific mutation subset is a welcome development, offering hope for improved outcomes.
- Diagnostic Challenges and Opportunities: While genomic testing is becoming more accessible in India, widespread and timely access to FDA-approved companion diagnostics like the Oncomine™ Dx Express Test remains a challenge, particularly in public healthcare settings or smaller cities. This approval highlights the need for increased investment in and standardization of molecular diagnostics across the country. To overcome this, collaboration between government health initiatives, private diagnostic labs, and pharmaceutical companies will be essential to establish robust and affordable testing infrastructure. Tele-pathology and centralized testing hubs could play a significant role in bridging geographical gaps.
- Affordability and Access: The cost of targeted therapies can be a significant barrier in India. Healthcare professionals and policymakers will need to address issues of affordability, reimbursement mechanisms, and patient assistance programs to ensure equitable access to ZEGFROVY. This may involve exploring tiered pricing models, local manufacturing partnerships, and government subsidies or insurance schemes to make the drug accessible to a wider patient base, including those in lower-income brackets.
- Physician Education and Awareness: Continuous medical education programs will be vital to raise awareness among oncologists and pathologists across India about EGFR exon 20 insertion mutations, the importance of testing, and the appropriate use and management of ZEGFROVY. These programs should leverage digital platforms, workshops, and collaborations with national oncology societies to disseminate the latest clinical evidence and practical guidance effectively.
- Real-World Evidence: Given the diverse patient population and healthcare infrastructure in India, the collection of real-world evidence on ZEGFROVY’s efficacy and safety in the Indian context will be valuable. Establishing patient registries and observational studies will be crucial to understand the drug’s performance in a real-world setting, potentially informing local clinical guidelines and optimizing treatment strategies tailored to the Indian population.
- Regulatory Alignment: While the FDA approval is a strong indicator, local regulatory approval from the Central Drugs Standard Control Organization (CDSCO) in India will be required for ZEGFROVY’s market entry. This process may involve additional data requirements or local clinical trials, which could impact the timeline for availability.
In both regions, the advent of ZEGFROVY necessitates a collaborative approach involving oncologists, pathologists, pharmacists, and patient support groups to ensure optimal patient identification, treatment, and management.
Key Takeaways for Clinicians
For healthcare professionals considering ZEGFROVY for their patients, several key points summarize the critical aspects of this new therapy:
- Targeted Therapy for a Specific Mutation: ZEGFROVY is indicated exclusively for NSCLC patients with confirmed EGFR exon 20 insertion mutations who have progressed on platinum-based chemotherapy. Accurate molecular testing is non-negotiable for patient selection.
- Promising Efficacy: Clinical trials demonstrated a notable Overall Response Rate (ORR) of 46% and a median Duration of Response (DOR) of 11.1 months, offering a significant improvement over historical outcomes for this challenging mutation.
- Mandatory “With Food” Administration: Adherence to taking ZEGFROVY with food is crucial for managing gastrointestinal side effects and ensuring consistent drug exposure.
- Proactive Toxicity Management: Clinicians must be vigilant for key adverse events, particularly ILD/pneumonitis, severe gastrointestinal issues, dermatologic reactions, and ocular toxicity. Early identification and adherence to dosage modification guidelines are paramount for patient safety and treatment continuity.
- Reproductive Counseling: Due to embryo-fetal toxicity and potential infertility, comprehensive counseling on effective non-hormonal contraception for both male and female patients of reproductive potential is essential.
- Drug-Drug Interactions: Awareness of potential interactions with strong CYP3A inhibitors/inducers and hormonal contraceptives is critical to prevent efficacy loss or increased toxicity.
- Accelerated Approval Context: While approved, clinicians should be aware that continued approval is contingent on confirmatory trials, emphasizing the evolving nature of data for this therapy.
Future Outlook and Ongoing Research
The accelerated approval of ZEGFROVY signifies a critical step forward, but the journey of a new drug does not end there. The future outlook for sunvozertinib involves several key areas:
- Confirmatory Trials: As part of the accelerated approval, ongoing or planned confirmatory trials will be crucial to solidify ZEGFROVY’s long-term clinical benefit, particularly in terms of overall survival. These studies will provide more robust data to support full approval and further define its role in the treatment algorithm.
- Earlier Lines of Therapy: Research may explore the potential use of sunvozertinib in earlier lines of therapy for EGFR exon 20 insertion mutation-positive NSCLC, potentially as a first-line treatment, either as monotherapy or in combination with other agents.
- Combination Therapies: Investigations into combination strategies with other targeted therapies, chemotherapy, or immunotherapy could further enhance efficacy and overcome potential resistance mechanisms.
- Resistance Mechanisms: Understanding and overcoming acquired resistance to ZEGFROVY will be a key area of future research, leading to the development of subsequent treatment strategies. This includes identifying novel mutations or bypass pathways that may emerge during treatment and developing strategies to counteract them.
- Global Expansion: Beyond the US, Dizal will likely pursue regulatory approvals in other key markets, including India, Europe, and other Asian countries, to make ZEGFROVY accessible to a broader global patient population.
- Biomarker Refinement: Further research into predictive biomarkers beyond the initial EGFR exon 20 insertion mutations could help refine patient selection and identify those most likely to respond, leading to even more personalized treatment approaches.
Conclusion
The FDA approval of ZEGFROVY (sunvozertinib) represents a significant advancement in the targeted treatment of locally advanced or metastatic non-small cell lung cancer with EGFR exon 20 insertion mutations. This patient population has historically faced limited effective therapeutic options, making ZEGFROVY a welcome addition to the oncology armamentarium.
Its targeted mechanism of action, compelling clinical trial results demonstrating significant overall response rates and durable responses, and a manageable safety profile underscore its potential to improve outcomes for eligible patients. While the accelerated approval pathway highlights the urgent unmet need and provides early access, ongoing confirmatory trials will be essential to further solidify its long-term benefits.
For healthcare professionals in India and the US, the introduction of ZEGFROVY necessitates a renewed focus on comprehensive genomic profiling for NSCLC patients, meticulous adherence to dosage and administration guidelines, and proactive management of potential adverse events. As precision oncology continues to evolve, ZEGFROVY stands as a testament to the power of targeted therapy in transforming the lives of patients battling complex cancers. Its arrival marks a new horizon, offering hope and a more tailored approach for those affected by this challenging form of lung cancer.
References:
- WU-KONG1. Assessing an Oral EGFR Inhibitor, Sunvozertinib in Patients Who Have Advanced Non-small Cell Lung Cancer with EGFR or HER2 Mutation (WU-KONG1)-ClinicalTrials.gov-(2025-02-28)
- U.S. Food and Drug Administration. ZEGFROVY (sunvozertinib) tablets, for oral use. Full Prescribing Information. Initial U.S. Approval: 2025
- U.S. Food and Drug Administration. Approval Letter for ZEGFROVY (sunvozertinib). [02.07.2025]. [FDA website]