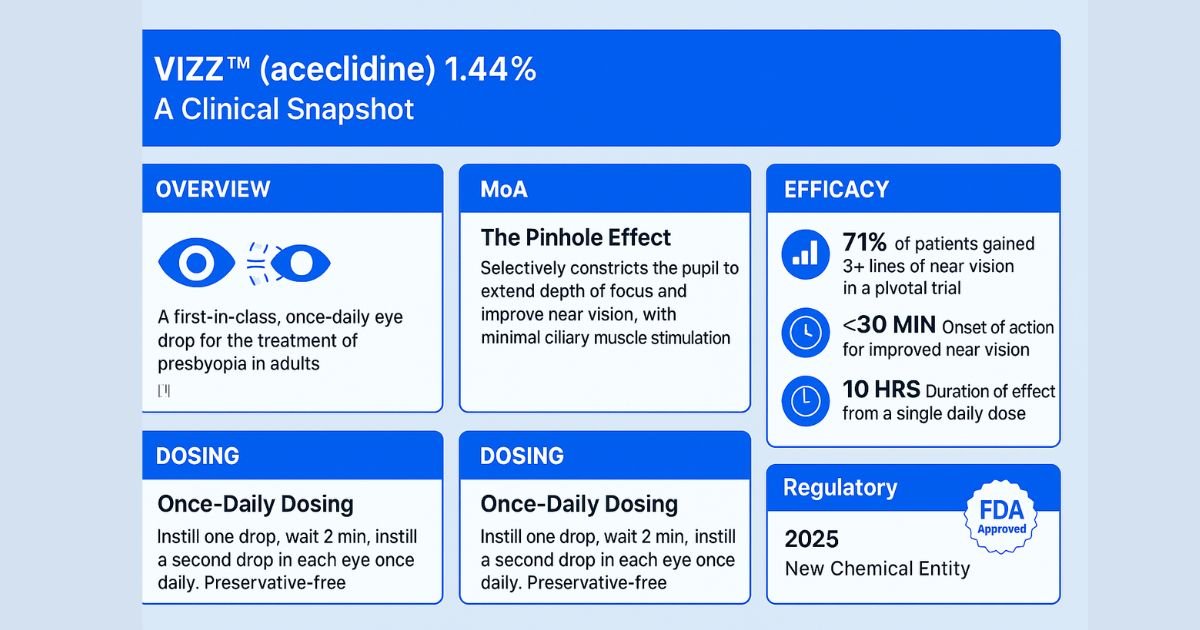
On 2025-07, the U.S. Food and Drug Administration (FDA) approved VIZZ aceclidine ophthalmic solution 1.44%, a first-in-class topical therapy for presbyopia [1]. Developed by LENZ Therapeutics, Inc., VIZZ is a novel cholinergic muscarinic agonist that offers a non-surgical, pharmacological approach to managing age-related loss of near vision. Its approval as a new chemical entity in the United States marks a pivotal development in ophthalmic therapeutics, providing a differentiated mechanism of action from existing treatments [2]. VIZZ is a sterile, preservative-free solution supplied in single-dose vials for once-daily topical administration [1]. This article provides a comprehensive review of the clinical evidence, mechanism of action, safety profile, and regulatory milestones for VIZZ, intended for ophthalmologists, optometrists, and other healthcare professionals.
Indications and Patient Selection
VIZZ is specifically indicated for the treatment of presbyopia in adults [1]. Patient selection should be guided by a confirmed diagnosis of presbyopia in adult patients seeking an alternative to corrective lenses or surgical intervention.
The pivotal CLARITY-1 and CLARITY-2 trials enrolled a broad population of adults aged 45 to 75 years with a refractive range from -4.00 to +1.00 D sphere and astigmatism up to 2.00 D [1]. This suggests VIZZ is suitable for a wide range of presbyopic patients, including those who are emmetropic, myopic, or hyperopic. Notably, the trials also included participants who were post-refractive surgery and/or pseudophakic, indicating potential utility in these complex patient populations [1].
Prior to initiation, a thorough ophthalmic examination is advised, including an examination of the retina, due to the rare risk of retinal tears associated with miotics [1].

Dosing and Administration
The recommended dosing for VIZZ is specific and designed for optimal efficacy.
- Dosage Regimen: Instill one drop in each eye, wait 2 minutes, then instill a second drop in each eye. This entire sequence is performed once daily from the same single-dose vial [1].
- Dosage Form: VIZZ is a 1.44% aceclidine solution (equivalent to 1.75% aceclidine hydrochloride) supplied in 0.4 mL single-dose vials [1].
- Administration Instructions:
- Contact Lenses: Patients should remove contact lenses prior to instillation and wait 10 minutes before reinserting them [1].
- Multiple Ophthalmic Products: If other topical ophthalmic medications are used, they should be administered at least 5 minutes apart [1].
- Aseptic Technique: To prevent contamination and injury, patients must be counseled to avoid touching the vial tip to the eye or any other surface. The single-dose vial and any remaining solution should be discarded immediately after use [1].
- Storage: VIZZ must be stored under refrigeration at 2°C to 8°C (36°F to 46°F). Once a pouch is opened or vials are removed from refrigeration, they may be stored at room temperature (up to 25°C/77°F) but must be used within 30 days [1].
Mechanism of Action
VIZZ’s therapeutic effect is derived from its activity as a cholinergic muscarinic agonist [1]. Aceclidine stimulates muscarinic receptors located on the smooth muscles of the eye. The key to its clinical profile is its selectivity. The FDA label describes VIZZ as a “predominately pupil selective miotic that interacts with the iris with minimal ciliary muscle stimulation” [1].
This selective action on the iris sphincter muscle causes it to contract, resulting in miosis (pupil constriction). This reduction in pupil diameter creates a “pinhole effect,” an optical phenomenon that increases the depth of focus. By extending the depth of focus, the eye is better able to focus light from near objects onto the retina, thereby improving near visual acuity without requiring accommodation from the crystalline lens [1]. This targeted mechanism, which minimizes effects on the ciliary body, is designed to reduce the side effects commonly associated with less selective miotics, such as accommodative spasm and brow ache.
Clinical Evidence
The approval of VIZZ was supported by a robust Phase 3 program, including two pivotal, randomized, double-masked, controlled studies: CLARITY-1 and CLARITY-2, and a long-term safety study, CLARITY-3 [1][3].
CLARITY-1 (NCT05656027)
CLARITY-1 was a 42-day study that randomized 313 participants to receive either VIZZ (N=157) or a brimonidine control (N=156). The primary efficacy endpoint was the proportion of participants gaining 3 lines or more in high contrast, distance-corrected near visual acuity (DCNVA) at 40 cm, without losing 1 line or more of distance-corrected distance visual acuity (DCDVA) at 4 meters, measured at Day 1, Hour 3 [1][3].
Results: The study met its primary endpoint with high statistical significance. A substantially greater proportion of participants in the VIZZ arm achieved the endpoint compared to the control arm.
Table 1: Primary Efficacy Endpoint Results for CLARITY-1 (Day 1, Hour 3)
| Treatment Group | N | Proportion Achieving Endpoint | p-value |
| VIZZ™ 1.44% | 157 | 65% | <0.01 |
| Brimonidine (Control) | 156 | 12% |
Source: VIZZ™ Full Prescribing Information [1][3]
The study also demonstrated a rapid onset of action, with significant improvements in near vision observed as early as 30 minutes post-dose, and a durable effect lasting up to 10 hours [1, 2,3].
CLARITY-2 (NCT05728944)
CLARITY-2 followed a similar 42-day design, randomizing 153 participants to receive either VIZZ (N=77) or a vehicle control (N=76). The primary endpoint was identical to that of CLARITY-1 [1][4].
Results: CLARITY-2 also met its primary endpoint, confirming the efficacy findings of CLARITY-1.
Table 2: Primary Efficacy Endpoint Results for CLARITY-2 (Day 1, Hour 3)
| Treatment Group | N | Proportion Achieving Endpoint | p-value |
| VIZZ™ 1.44% | 77 | 71% | <0.01 |
| Vehicle (Control) | 76 | 8% |
Source: VIZZ™ Full Prescribing Information [1][4]
CLARITY-3 (NCT05753189)
CLARITY-3 was a 6-month, randomized, double-masked, controlled study designed to evaluate the long-term safety of VIZZ in 217 participants. The results from this study supported the safety profile established in the shorter-term trials, indicating that the majority of adverse reactions were mild, transient, and self-resolving [1][5].
Safety Profile
The safety of VIZZ was evaluated in over 680 participants across the Phase 3 program [1]. The overall profile is favorable, with no boxed warnings, REMS, or contraindications.
- Common Adverse Reactions: The most frequently reported adverse reactions (≥5%) in the pivotal trials were primarily ocular and mild in nature.
- Instillation site irritation: 20%
- Dim vision: 16%
- Headache: 13%
- Conjunctival hyperemia: 8%
- Ocular hyperemia: 7% [1]
- Serious Adverse Events: No serious adverse events were observed across the clinical trial program [2].
- Warnings and Precautions: The label includes several key warnings:
- Blurred Vision: Patients may experience temporary blurred or dim vision and should not drive or operate machinery if vision is affected [1].
- Risk of Retinal Tear/Detachment: As a miotic, VIZZ carries a rare risk of retinal tears. A retinal examination is advised prior to therapy initiation [1].
- Iritis: Caution is advised in patients with a history of iritis [1].
Use in Special Populations
- Pregnancy and Lactation: There are no adequate and well-controlled studies in pregnant or lactating women. Systemic exposure to aceclidine is low following topical administration, but it should be used during pregnancy only if the potential benefit justifies the potential risk [1].
- Pediatric Use: Presbyopia does not occur in the pediatric population, so safety and effectiveness have not been established [1].
- Geriatric Use: No overall differences in safety or efficacy were observed between patients aged 65 and older and younger adult patients [1].
Drug Interactions and Contraindications
- Drug Interactions: No formal drug interaction studies have been conducted. The only guidance provided is to separate the administration of VIZZ and other topical ophthalmic products by at least 5 minutes [1].
- Contraindications: There are no contraindications for VIZZ [1].
Pharmacokinetics and Pharmacodynamics
- Pharmacokinetics (PK): Aceclidine is hydrolyzed in the eye to its metabolite, 3-quinuclidinol (3-Q). Systemic PK studies measured 3-Q levels. Following 8 days of once-daily dosing in 16 subjects, the mean Cmax for 3-Q was 2.114 ng/mL and the mean AUC0-t was 4.899 hr*ng/mL. There was little to no accumulation with repeat dosing [1].
- Pharmacodynamics (PD): The pharmacodynamic properties were not explicitly detailed in the label, but the mechanism of action—miosis via iris sphincter contraction—is the primary pharmacodynamic effect leading to improved near vision [1].
Regulatory Path and Milestones
- FDA Approval: VIZZ was approved by the U.S. FDA on July 07, 2025 under Application Number 218585 [1].
- Regulatory Designation: It is considered a New Chemical Entity (NCE) in the United States, signifying its novel active ingredient [2].
- Other Designations: UNKNOWN. The provided sources do not mention if VIZZ received Fast Track, Breakthrough Therapy, or Priority Review designations.
Global Context (EMA/CDSCO)
- European Medicines Agency (EMA): UNKNOWN. Publicly available information regarding a Marketing Authorisation Application (MAA) to the EMA for aceclidine for presbyopia is not available as of 2025-08-18.
- Central Drugs Standard Control Organisation (CDSCO) of India: UNKNOWN. There is no publicly available information regarding an application or approval for VIZZ in India as of 2025-08-18.
Practical Considerations for Clinicians
VIZZ offers a significant new tool for presbyopia management. Key considerations for integration into practice include patient education on the expected side effects, such as temporary dim vision and instillation site irritation. Proper counseling on administration technique, especially the 2-minute wait between drops and handling of the single-dose vial, is crucial for efficacy and safety. The lack of contraindications and a preservative-free formulation are notable benefits. The need for refrigeration may present a logistical consideration for some patients.
Limitations and Evidence Gaps
While the Phase 3 data are robust, some evidence gaps remain. The provided data by FDA Assessdata does not include head-to-head comparisons against other pharmacological agents for presbyopia, such as pilocarpine. Long-term safety and efficacy data beyond the 6-month CLARITY-3 study will be valuable to collect through real-world evidence and post-marketing surveillance.
The efficacy in specific subpopulations, such as those with prior corneal or lenticular surgery, was included but not detailed separately in the provided label.
Patient-Friendly Summary
VIZZ™ (aceclidine ophthalmic solution) 1.44% for Presbyopia
VIZZ™ is a new, FDA-approved eye drop used once a day to treat presbyopia, the blurry near vision that happens to most people as they get older. It is for adults who want to improve their near vision without needing reading glasses all the time.
VIZZ works by making your pupil smaller. This creates a “pinhole effect” that helps your eye focus better on close-up objects, like your phone or a menu. The effect starts working within 30 minutes and can last for up to 10 hours.
In clinical studies, up to 7 out of 10 people who used VIZZ had a significant improvement in their near vision. The most common side effects were temporary and included mild eye irritation, dim vision, and headache. VIZZ is preservative-free and has no contraindications, meaning it is suitable for a wide range of adults. If you are tired of reaching for your reading glasses, talk to your eye doctor to see if VIZZ is right for you. Do not drive if your vision is blurry after using the drops.
Frequently Asked Questions
What is VIZZ™?
VIZZ™ (aceclidine ophthalmic solution) 1.44% is a new, once-daily prescription eye drop FDA-approved to treat presbyopia (age-related blurry near vision) in adults.
How long does it take for VIZZ to work?
VIZZ has a rapid onset of action, with effects on near vision beginning within 30 minutes of administration.
Can patients drive after using VIZZ?
The official prescribing information advises patients not to drive or operate machinery if their vision is not clear. This is due to potential temporary side effects like blurred or dim vision. Patients should also exercise caution when driving at night.
What are the most common side effects of VIZZ?
The most common side effects are typically mild and transient, including instillation site irritation (20%), temporary dim vision (16%), and headache (13%).
Is VIZZ a permanent cure for presbyopia?
No. VIZZ is a daily treatment, not a cure. Its effects last for up to 10 hours, after which near vision will return to its baseline state. It provides a temporary, pharmacological solution to manage the symptoms of presbyopia.
References
[1] VIZZ™ (aceclidine ophthalmic solution) 1.44% Full Prescribing Information. LENZ Therapeutics, Inc. Reference ID: 5635114. Revised: 2025-07.-[PRESCRIBING INFORMATION OF VIZZ]
[2] Briefing Document: VIZZ™ (Aceclidine Ophthalmic Solution) 1.44% for Presbyopia Treatment. 2025-08-16.
[3] Phase 3 Evaluation of the Safety and Efficacy of LNZ101 for the Treatment of Presbyopia (CLARITY-1)
[4] Phase 3 Efficacy Study of LNZ101 for the Treatment of Presbyopia (CLARITY-2)
[5] Phase 3 Safety Study for the Treatment of Presbyopia Subjects(CLARITY-3)