Grafapex Treosulfan – A New Era in Stem Cell Transplantation
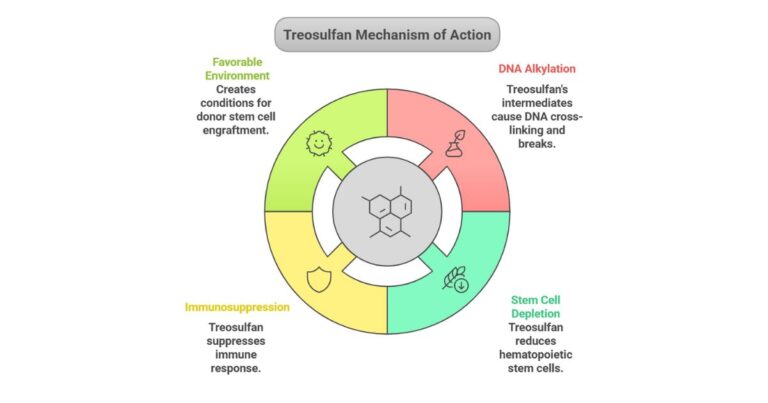
The U.S. Food and Drug Administration (FDA) approved Grafapex treosulfan on January 22, 2025, marking a significant advancement in preparative regimens for allogeneic hematopoietic stem cell transplantation (alloHSCT) in patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) 310. Developed by Medac GmbH and…
