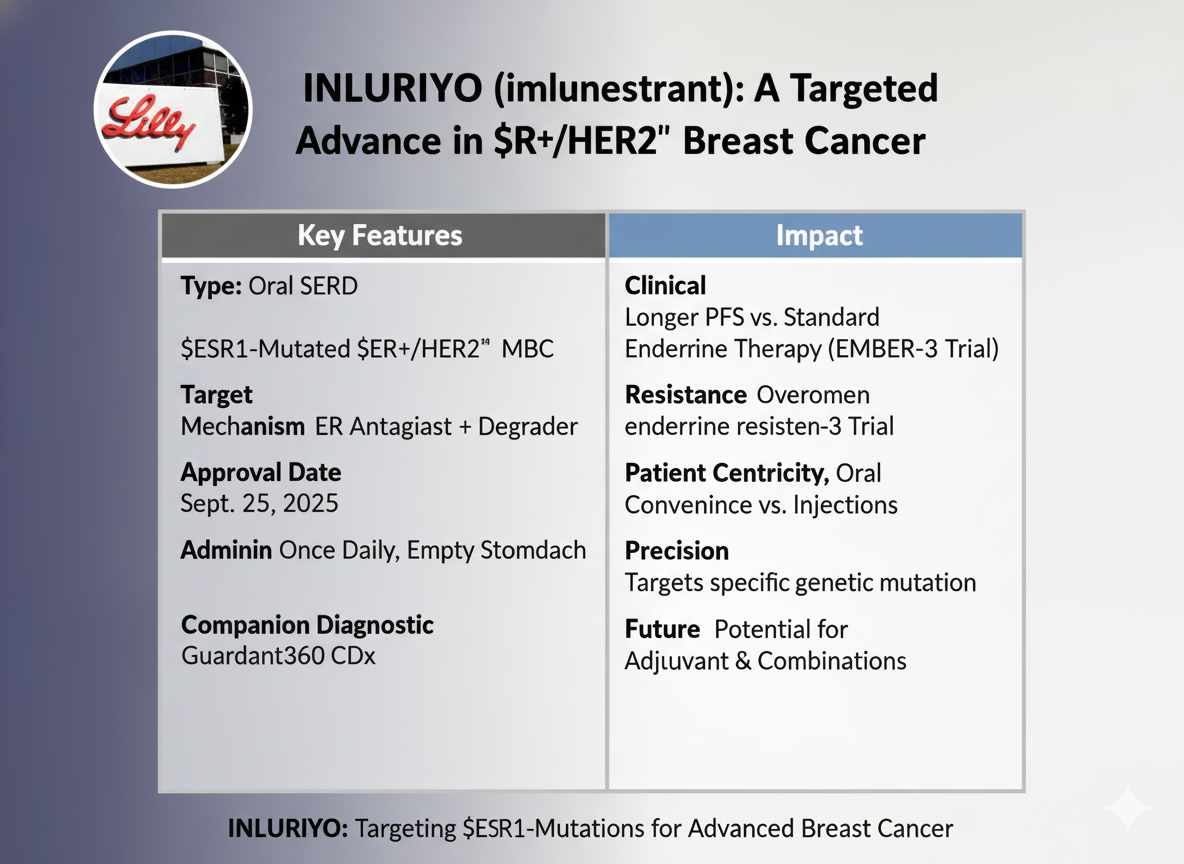
The landscape of endocrine therapy for hormone receptor-positive (HR+) breast cancer continues to evolve rapidly, particularly for patients whose disease has become resistant to standard-of-care treatments. On September 25, 2025, the FDA approved INLURIYO (imlunestrant) tablets, a new oral selective estrogen receptor antagonist and degrader (SERD). This marks an important milestone for patients with estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative, ESR1-mutated advanced or metastatic breast cancer whose disease has progressed following at least one line of endocrine therapy.
INLURIYO’s approval is based on its demonstrated ability to overcome a key mechanism of endocrine resistance, offering a statistically significant improvement in progression-free survival (PFS) and the convenience of an all-oral regimen.
Background & Disease Setting
Breast cancer, the most common cancer in women globally, is often classified by the expression of receptors. The ER+ HER2– subtype represents approximately 70% of all breast cancer cases. While hormonal therapies, such as Aromatase Inhibitors (AIs), are highly effective initially, advanced or metastatic disease is considered incurable, and patients inevitably develop resistance.
The Challenge of ESR1 Mutations
A primary driver of this acquired resistance, particularly after exposure to AIs, is the emergence of activating missense mutations and in-frame deletions in the ligand-binding domain (LBD) of the ESR1 gene.
- Prevalence: ESR1 mutations are estimated to occur in 10% to 50% of ER+ HER2– advanced or metastatic breast cancers following prior endocrine therapy.
- Mechanism: These mutations lead to the constitutive activation of the estrogen receptor (ER), allowing the tumor to grow independently of estrogen signaling. This effectively renders traditional AIs largely ineffective.
Current Treatment Landscape
Before INLURIYO, therapeutic options in this setting were primarily limited to:
- Fulvestrant (an injectable SERD): Often limited by patient discomfort due to its intramuscular injection route and demonstrated inferior performance after progression on a CDK4/6 inhibitor therapy.
- Exemestane (a steroidal AI).
- Elacestrant (the first approved oral SERD for this indication in January 2023).
- Other targeted agents (e.g., PI3K/AKT/mTOR inhibitors) or chemotherapy.
The ongoing need has been for new, tolerable, and orally bioavailable agents that can effectively target the mutated ER and overcome this key resistance mechanism.
Scientific Detail: Mechanism and Pharmacology
Mechanism of Action (MoA)
INLURIYO (imlunestrant) is a next-generation oral Selective Estrogen Receptor Antagonist (SERA) and Degrader (SERD). It works through a dual mechanism to disrupt the ER-driven growth signal, effectively targeting the challenge posed by ESR1 mutations.
- ER Antagonism: It binds to both wild-type and mutant ERα, competitively blocking estrogen from binding and preventing ER-dependent gene transcription.
- Receptor Degradation: It rapidly induces the degradation of the ERα protein, thereby achieving continuous ER inhibition and degradation, a key step in overcoming resistance from constitutively active ESR1 mutations.
Pharmacokinetics (PK)
- Absorption: Absolute oral bioavailability is low at 10%. The median time to maximum plasma concentration (Tmax) is 4 hours.
- Distribution: Highly protein bound (>99%). The apparent volume of distribution is large at 8,120 \L.
- Elimination: The elimination half-life (t1/2) is approximately 30 hours, supporting the once-daily (QD) dosing regimen. Elimination is primarily via feces (97% of dose, with 62% of the dose being unchanged drug).
- Metabolism: Metabolized by sulfation, CYP3A4, and direct glucuronidation (UGT1A1, 1A3, 1A8, 1A9, 1A10).
Clinical Use & Administration
Patient Selection and Companion Diagnostic
- Patient Selection: Treatment with INLURIYO is specifically for adult patients with ER+\HER2– advanced or metastatic breast cancer that harbor ESR1 mutations.
- Companion Diagnostic: Patient selection must be based on the presence of an ESR1 mutation(s) in a plasma specimen using an FDA-approved test. The FDA granted a simultaneous approval to the Guardant 360 CDx assay as the companion diagnostic device for INLURIYO.
Dosing and Administration
- Recommended Dosage: 400 mg orally once daily (two 200mg tablets) until disease progression or unacceptable toxicity.
- Food Effect: Must be taken on an empty stomach, defined as at least 2 hours before food, or 1 hour after food. This is critical as administration with a low-fat meal increased imlunestrant C_max by 3.6-fold and AUC by 2-fold.
- Tablet Handling: Swallow the tablets whole. Do not split, crush, or chew them.
- Missed Dose/Vomiting: If a dose is missed by 6 or more hours or if the patient vomits, the patient should skip the dose and take the next dose the following day at the regularly scheduled time.
Dosage Modifications for Special Populations & Drug Interactions
| Patient Population | Recommended Dosage Modification | Clinical Rationale/Implication |
| Hepatic Impairment (Moderate Child-Pugh B or Severe Child-Pugh C) | Reduce dose to 200 mg once daily. | AUC increased 2.2-fold in moderate, and 3.1-fold in severe hepatic impairment; reduction maintains exposure within a safe margin. |
| Strong CYP3A Inhibitors (e.g., itraconazole, ketoconazole) | Avoid concomitant use. If unavoidable, decrease dose to 200mg once daily. | Inhibitor increases imlunestrant AUC 2.1-fold and C_max 1.9-fold, increasing risk of INLURIYO associated adverse reactions. |
| Strong CYP3A Inducers (e.g., carbamazepine, rifampin) | Avoid concomitant use. If unavoidable, increase dose to 600mg once daily. | Inducer decreases imlunestrant AUC by 42% and Cmax by 29%, potentially reducing effectiveness. |
| P-gp or BCRP Substrates (e.g., digoxin, rosuvastatin) | Avoid concomitant use unless dosage modification is recommended for the substrate. | INLURIYO inhibits both transporters, increasing substrate exposure and risk of adverse reactions related to the substrate. |
Clinical Development and Efficacy
EMBER-3 Pivotal Trial
The approval is primarily based on results from the EMBER-3 (J2J-OX-JZLC) trial, a Phase 3, randomized, open-label, active-controlled, multicenter study that enrolled 874 adult patients with ER+ HER2– locally advanced or metastatic breast cancer.
- Patient Population: The approved indication focuses on the cohort with ESR1-mutated tumors (n=256). These patients had disease progression following prior endocrine therapy (AI pm CDK 4/6i).
- Randomization Arms (for primary efficacy):
- Arm A: INLURIYO 400mg QD monotherapy.
- Arm B (Control): Investigator’s choice of endocrine therapy (fulvestrant or exemestane, referred to as TPC or SOC).
- Primary Endpoint: Investigator-assessed Progression-Free Survival (PFS) in the ESR1-mutated population.
Key Efficacy Results (ESR1-Mutated Population)
INLURIYO demonstrated a statistically significant and clinically meaningful improvement in PFS.
| Efficacy Endpoint | INLURIYO (n=138) | Fulvestrant or Exemestane (n=118) | Hazard Ratio (95% CI); p-value |
| Median PFS (Investigator-Assessed) | 5.5 months (95% CI: 3.9, 7.4) | 3.8 months (95% CI: 3.7, 5.5) | 0.62 (95% CI: 0.46, 0.82); mathbf0.0008 |
| Objective Response Rate (ORR) | 14.3% (95% CI: 7.8, 20.8) | 7.7% (95% CI: 2.2, 13.2) | N/A |
| Overall Survival (OS) | Immature data (31% maturity) | Immature data (31% maturity) | HR 0.55 (95% CI: 0.35, 0.86) – Favorable trend observed |
The HR of 0.62 signifies a 38% reduction in the risk of disease progression or death compared to standard endocrine therapy.
- PFS Robustness: The benefit was consistently observed in the Blinded Independent Review Committee (BIRC)-assessed PFS, lending confidence to the investigator-assessed results despite the open-label design.
- Subpopulation: Although no male patients were enrolled in the ESR1-mutated cohort, the FDA approved the indication for adult males based on the biological mechanism of action and extrapolation from female patient data.
Efficacy & Safety
Comprehensive Adverse Event Profile
The safety profile of INLURIYO was generally manageable, largely consisting of Grade 1-2 events typical of an endocrine therapy.
| Adverse Reaction (≥10% Incidence in INLURIYO arm) | INLURIYO (All Grades) | INLURIYO (Grades 3 or 4) | Fulvestrant or Exemestane (All Grades) | Fulvestrant or Exemestane (Grades 3 or 4) |
| Hemoglobin decreased (Lab Abnormality) | 30% | 1.2% | 35% | 3.4% |
| Musculoskeletal Pain | 30% | 3.7% | 29% | 1.9% |
| Calcium decreased (Lab Abnormality) | 26% | 0% | 19% | 0.6% |
| Neutrophils decreased (Lab Abnormality) | 26% | 4% | 29% | 4.7% |
| AST increased (Lab Abnormality) | 25% | 1.9% | 27% | 2.3% |
| Fatigue | 23% | 0.3% | 14% | 0.6% |
| Diarrhea | 22% | 0.6% | 12% | 0.0% |
| ALT increased (Lab Abnormality) | 21% | 1.3% | 23% | 1.0% |
| Triglycerides increased (Lab Abnormality) | 21% | 0% | 22% | 1.2% |
| Nausea | 17% | 0.3% | 13% | 0.0% |
| Platelets decreased (Lab Abnormality) | 16% | 1.8% | 14% | 1.3% |
| Constipation | 10% | 0% | 6% | 0.3% |
| Cholesterol increased (Lab Abnormality) | 10% | 0% | 12% | 0% |
| Abdominal pain | 10% | 0.3% | 6% | 0.6% |
- Gastrointestinal (GI) Toxicities: The most notable numerical increases in adverse reactions for INLURIYO compared to the standard endocrine therapy arm were in GI events: diarrhea (22% vs. 12%), nausea (17% vs. 13%), constipation (10% vs. 6%), and abdominal pain (10% vs. 6%). Fatigue was also notably higher (23% vs. 14%).
- Serious Adverse Reactions (SARs) & Deaths: SARs occurred in 10% of patients on INLURIYO, comparable to the 11% in the control arm. The most common SAR (>1%) with INLURIYO was pleural effusion (1.2%). Fatal adverse reactions occurred in 1.8% of INLURIYO-treated patients, primarily due to disease progression, though cardiac arrest and upper GI hemorrhage were among the fatal events reported.
- Treatment Discontinuation: Permanent treatment discontinuation due to an adverse reaction occurred in 4.6% of patients on INLURIYO, compared to 1.2% in the control arm. The most frequent cause was increased ALT(0.9%).
Dose Modifications and Management
- Dose Reduction: The recommended dose reduction for adverse reactions is to 200 mg once daily. Permanent discontinuation is required if 200mg is not tolerated. Dose reductions were relatively low in the trial (2.4%).
- Hepatotoxicity: Liver transaminase elevations (AST\ALT) require dose suspension and potentially a dose reduction to 200mg QD, or permanent discontinuation in cases of severe liver injury (ALT or AST 3 times ULN concurrent with TBL 2 times ULN).
Clinical Use & Patient Counseling
Reproductive Health and Safety
The embryo-fetal toxicity risk is categorized in the Warnings and Precautions section.
- Contraception: Females of reproductive potential and male patients with female partners of reproductive potential must use effective contraception during treatment and for 1 week after the last dose. Pregnancy status must be verified prior to initiating therapy.
- Lactation: Women should not breastfeed during treatment and for 1 week after the last dose.
- Fertility: Based on animal data, INLURIYO may impair fertility in males and females, though findings were reversible in rats.
Dyslipidemia and Monitoring
Hypercholesterolemia and hypertriglyceridemia (dyslipidemia) occurred frequently, though at a similar rate to the control arm. Given the known class effect of endocrine therapies, the prescribing information advises patients that dyslipidemia may occur and requires lipid profile monitoring prior to starting treatment and periodically thereafter.
Unique Value & Impact
A Convenient Oral Option
The approval of INLURIYO reinforces the shift toward all-oral endocrine therapy in breast cancer. The oral route is a major improvement over the historical injectable SERD, fulvestrant, eliminating the burden of in-office intramuscular injections, which many patients find painful and burdensome.
Precision Medicine for Resistance
INLURIYO is a true precision oncology agent, explicitly indicated for patients whose tumors harbor the ESR1 mutation. The clear, predictable drug delivery and systemic exposure achieved by this oral SERD allow it to effectively address this specific mechanism of endocrine resistance. While elacestrant is a similar oral SERD, the availability of a second agent expands treatment choices for clinicians and patients, allowing for individual decisions based on tolerability and prior experience.
Future Directions
The EMBER-3 trial included an exploratory arm investigating INLURIYO in combination with abemaciclib (a CDK 4/6 inhibitor), which showed promising early results. Continued clinical investigation, notably in the ongoing Phase 3 EMBER-4 trial, is exploring the role of imlunestrant in the adjuvant setting for early breast cancer patients at increased risk of recurrence. This work aims to establish INLURIYO’s utility earlier in the disease course and potentially in combination with other targeted agents to redefine the standard of care.
“The EMBER-3 trial data are encouraging, showing that imlunestrant monotherapy provides clinical benefit in ESR1-mutated tumors. This offers another meaningful treatment option for patients after at least one line of endocrine therapy2.”
Check the Availability in India: Contact Us
FAQs
How does INLURIYO’s oral dosing impact patient quality of life compared to previous injectable SERDs like fulvestrant, especially for long-term treatment?
The shift to an oral formulation significantly enhances patient convenience and quality of life by eliminating the need for regular, often painful, intramuscular injections that fulvestrant requires. This can reduce clinic visits, improve adherence, and empower patients with greater autonomy over their treatment schedule, potentially easing the psychological burden associated with advanced cancer management. For many, the ability to take medication at home contributes to a more normal daily routine.
Given that INLURIYO specifically targets ESR1-mutated breast cancer, what are the implications for patients whose tumors do not have this mutation?
INLURIYO’s approval is specific to ESR1-mutated ER+ HER2– advanced breast cancer. For patients whose tumors lack this mutation, INLURIYO is not currently indicated. This highlights the growing importance of genomic testing, like the Guardant 360 CDx, to guide treatment decisions. In these cases, other endocrine therapies, CDK 4/6 inhibitors, or other targeted agents would be considered based on the tumor’s specific molecular profile and prior treatment history.
What is the significance of the “empty stomach” administration requirement for INLURIYO, and what practical advice can be given to patients to ensure compliance?
The empty stomach requirement (at least 2 hours before food, or 1 hour after food) is crucial because food significantly increases the drug’s absorption, which can lead to higher blood levels and a greater risk of side effects. To ensure compliance, patients should be counselled to establish a consistent routine, such as taking it first thing in the morning upon waking, or before bed. Using medication reminders and integrating it into their daily schedule can also be very helpful. Emphasizing the “why” (to minimize side effects and optimize efficacy) can also boost adherence.
How might the development of INLURIYO and similar oral SERDs influence the design of future clinical trials for ER+ HER2– breast cancer?
The success of INLURIYO and other oral SERDs targeting specific resistance mechanisms is likely to accelerate the trend towards biomarker-driven trial designs. Future trials will increasingly focus on genetically selected patient populations, allowing for more precise evaluation of novel agents. We may also see more trials exploring these oral SERDs in combination with other targeted therapies (e.g., CDK4/6 inhibitors, PI3K inhibitors) or even in earlier lines of therapy and the adjuvant setting, further personalizing treatment approaches based on the tumor’s evolving molecular landscape.
The article mentions that INLURIYO may impair fertility. What resources or discussions should healthcare providers offer to patients of reproductive age who are considering this treatment?
For patients of reproductive age, a thorough discussion about fertility preservation options (e.g., egg or embryo freezing for women, sperm banking for men) before starting INLURIYO is critical. Healthcare providers should facilitate referrals to fertility specialists and provide comprehensive counseling on the potential for reversible or irreversible fertility impact. Additionally, clear guidance on effective contraception throughout treatment and for 1 week after the last dose is essential for both male and female patients to prevent pregnancy due to potential embryo-fetal toxicity.
Summary
INLURIYO (imlunestrant) marks a pivotal advance for ESR1 mutated ER+ HER2– advanced breast cancer. This oral SERD offers improved PFS over standard therapy, targeting a key resistance mechanism. Its convenient daily dosing, precise action, and manageable safety profile position it as a significant new option, improving outcomes and quality of life for eligible patients.
References
- FDA Official Approval: Approval Letter for NDA 218881 [https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2025/218881Orig1s000ltr.pdf].
- Full Prescribing Information (USPI): Full Prescribing Information for INLURIYO (imlunestrant) Tablets [https://pi.lilly.com/us/inluriyo-uspi.pdf].
- Patient Information (PPI): Patient Information for INLURIYO (imlunestrant) Tablets [https://pi.lilly.com/us/inluriyo-us-ppi.pdf].
- Multi-Discipline Review: NDA/BLA Multi-disciplinary Review and Evaluation (Clinical, Statistical, and Clinical Pharmacology Sections) [https://www.accessdata.fda.gov/drugsatfda_docs/nda/2025/218881Orig1s000MultidisciplineR.pdf].
- Clinical Inspection Summary: Clinical Inspection Summary for NDA 218881 [https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2025/218881Orig1s000ltr.pdf].
- Proprietary Name Review: Proprietary Name Review(s) for NDA 218881 [https://www.accessdata.fda.gov/drugsatfda_docs/nda/2025/218881Orig1s000NameR.pdf].
- Clinical Pharmacology Review (QT Study): Interdisciplinary Review Team for Cardiac Safety Studies (QT Study Review) [https://www.accessdata.fda.gov/drugsatfda_docs/nda/2025/218881Orig1s000OtherR.pdf].
- Risk Assessment and Risk Mitigation Review (REMS): Division of Risk Management Evaluation of Need for a REMS [https://www.accessdata.fda.gov/drugsatfda_docs/nda/2025/218881Orig1s000RiskR.pdf].
- Product Quality Review (Chemistry): Product Quality Review (Integrated Quality Assessment Template) [https://www.accessdata.fda.gov/drugsatfda_docs/nda/2025/218881Orig1s000ChemR.pdf].