On January 12, 2026, the U.S. Food and Drug Administration (FDA) officially approved ZYCUBO® (copper histidinate) for injection, marking a historic achievement as the first and only approved treatment for Menkes disease in the United States. Developed by Sentynl Therapeutics, Inc. (a subsidiary of Zydus Lifesciences Ltd.), ZYCUBO addresses a decades-long clinical void for neonates and pediatric patients suffering from this lethal, X-linked recessive neurodegenerative disorder.
Before this approval, Menkes disease was considered universally fatal in its classical form, with a median life expectancy of just 17.6 months. The clinical significance of ZYCUBO lies in its ability to restore copper homeostasis and significantly extend life, particularly when initiated within the “golden window” of the first four weeks of birth. For healthcare providers (HCPs) and investors, this approval represents a paradigm shift in neonatal care and a successful validation of the orphan drug development pathway.
Clinical and regulatory data for ZYCUBO (copper histidinate) based on the FDA-approved labeling and strategic analysis documents:
| Field | Value |
| Generic Name | copper histidinate |
| Brand Name | ZYCUBO® |
| Dosage Form | Lyophilized powder for injection (2.9 mg/vial) |
| Route | Subcutaneous (SC) |
| Indication | Treatment of Menkes disease in pediatric patients |
| Mechanism | Bioavailable copper replacement that bypasses the impaired gastrointestinal absorption observed in Menkes disease |
| Top 3 Side Effects | Pneumonia (30%), viral infection (27%), and respiratory failure/seizure (23% each) |
| Contraindications | None |
| Trial Phase | Phase 1/2 (Pooled analysis of Trial 1 & Trial 2) |
| Trial N | 129 (Safety population); 83 (Efficacy: 66 treated, 17 external control) |
| Primary Endpoint Result | 78% reduction in risk of death; Median overall survival 177.1 months vs. 17.6 months in controls |
| Approval Date | January 12, 2026 |
| Manufacturer | Sentynl Therapeutics, Inc. (Manufactured by Zydus Lifesciences Ltd.) |
| Black Box Warning | NO |
| REMS Required | NO (Not specified in prescribing information or approval letter) |
| Cost (if available) | Not publicly disclosed (WAC pricing unavailable as of Jan 2026) |
Disease Context: Pathogenesis and Clinical Burden
Epidemiology and Genetics
Menkes disease is an ultra-rare multisystemic disorder affecting approximately 1 in 100,000 to 250,000 live births [Source: CheckRare]. It is caused by pathogenic mutations in the ATP7A gene, located on chromosome Xq21.1, which encodes a critical copper-transporting P-type ATPase.
The Mechanism of Deficiency
In healthy individuals, the ATP7A protein is responsible for two primary functions: exporting copper across the basolateral membrane of intestinal enterocytes into the blood and transporting copper across the blood-brain barrier. In Menkes disease, this protein is dysfunctional or absent, leading to:
- Intestinal Entrapment: Dietary copper becomes trapped within the intestinal lining, unable to reach the systemic circulation.
- Cerebral Deprivation: Even if copper reaches the blood, it cannot effectively enter the brain, leading to profound neurological impairment.
- Enzymatic Failure: Many critical enzymes require copper as a cofactor, including cytochrome c oxidase (for energy), lysyl oxidase (for connective tissue), and tyrosinase (for pigmentation).
Unmet Clinical Need
Untreated patients typically present with sparse, depigmented “kinky” hair (pili torti), severe hypotonia, seizures, and neurodevelopmental regression. Without intervention, death usually occurs by age 3 due to respiratory infection or vascular complications [Source: Epocrates].
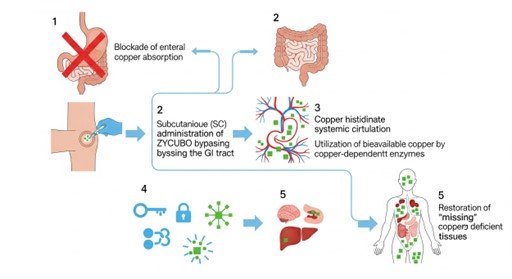
Mechanism of Action: The Subcutaneous Bypass
ZYCUBO (copper histidinate) functions as a bioavailable copper replacement therapy. The pharmacological goal is to restore the “missing” copper to deficient tissues and enzymes.
Molecular Target and Delivery
The primary challenge in Menkes disease is the blockade of enteral absorption. ZYCUBO is specifically formulated for subcutaneous (SC) administration, which bypasses the gastrointestinal tract entirely. By delivering copper histidinate into the subcutaneous tissue, the mineral enters the systemic circulation directly, where it can be utilized by copper-dependent enzymes throughout the body.
Layperson Analogy: > Imagine the body’s copper supply is a shipment of fuel. In Menkes disease, the main “unloading dock” (the intestines) is broken, so no fuel can get in. ZYCUBO acts as a “biological bypass,” delivering the fuel via a different entrance (the skin), ensuring the brain and heart can continue to run.
Therapeutic Landscape: Comparative Analysis
Prior to ZYCUBO’s approval, the therapeutic landscape was fragmented and lacked regulatory oversight.
| Feature | Untreated/Standard Support | ZYCUBO (Copper Histidinate) |
| Approval Status | None | FDA-Approved (Jan 2026) |
| Median Survival | 17.6 months | 177.1 months (Early Treatment) |
| Neurodevelopment | Severe, rapid decline | Improved outcomes with early start |
| Method | Palliative care | Subcutaneous injection (1-2x daily) |
| Availability | Supportive only | Specialty Pharmacy / Regulated Supply |
While off-label copper acetate or glycinate injections were sometimes used historically, they often lacked the stability and physiological pH found in ZYCUBO, which is designed to improve local tolerability and systemic bioavailability.
Clinical Trial Evidence: Redefining Survival
The efficacy of ZYCUBO was established through a pooled analysis of two open-label, single-arm, multicenter trials (Trial 1, NCT00001262 and Trial 2, NCT00811785).
Trial Design and Methodology
- Population: 66 treated pediatric patients with Menkes disease vs. 17 external contemporaneous controls.
- Duration: Treatment up to 3 years with long-term survival follow-up.
- Cohorts: Patients were stratified into an Early Treatment (ET) group (initiated within 4 weeks of birth) and a Late Treatment (LT) group (initiated after 4 weeks).
Primary Endpoint: Overall Survival (OS)
The primary endpoint was overall survival from birth compared to an untreated historical control cohort.
Key Statistical Findings (Early Treatment Cohort):
- Median OS: 177.1 months (95% CI: 33.0, NE) for ZYCUBO-ET vs. 17.6 months (95% CI: 11.5, 28.6) for Untreated Control.
- Hazard Ratio (HR): 0.22 (95% CI: 0.10, 0.49).
- p-value: p < 0.0001.
- Clinical Impact: Treatment with ZYCUBO was associated with a 78% reduction in the risk of death compared to no treatment.
Late Treatment Findings:
Even in patients starting therapy after 4 weeks, a survival benefit was observed, with a median OS of 62.4 months (HR: 0.27, p < 0.0001).
Neurodevelopmental Outcomes
Secondary analyses indicated that patients receiving early treatment showed significantly better brain growth, as measured by head circumference Z-scores (p = 0.0009), and some patients were able to attend school and participate in active play.
Safety Profile: Monitoring Copper Accumulation
While ZYCUBO is life-saving, its therapeutic index is narrow. The safety population (N=129) demonstrated that copper toxicity is a critical management priority.
BLACK BOX WARNING: NONE.
Adverse Reactions (Incidence ≥ 7%)
The most common adverse reactions reported in clinical trials include:
- Pneumonia: 30%
- Viral Infection: 27%
- Respiratory Failure: 23%
- Seizures: 23%
- Bacterial Infection: 20%
- Anemia: 9%
Warnings and Precautions
- Kidney and Liver Toxicity: Chronic copper accumulation can lead to renal tubular toxicity (proteinuria, acidosis) and elevations in liver transaminases. Preclinical data in juvenile rats showed single-cell necrosis and fibrosis at high doses.
- Hematological Impact: Anemia can occur due to interference with iron metabolism.
- Specific Populations: Infants under 2 years are at higher risk of toxicity due to immature renal and hepatic function.
Required Monitoring Protocol
HCPs must strictly adhere to the following monitoring schedule:
- Baseline: Serum copper, ceruloplasmin, electrolytes, LFTs, and CBC.
- Ongoing: Every 6 weeks for the first 6 months.
- Maintenance: Every 3 months for the next 18 months, then every 6 months thereafter.
Practical Dosing and Administration
ZYCUBO is provided as a 2.9 mg single-dose vial of lyophilized powder.
Standard Regimen
| Age Group | Dose | Frequency |
| Birth to < 1 Year | 1.45 mg | Twice Daily (8-12 hours apart) |
| 1 Year to < 17 Years | 1.45 mg | Once Daily |
Administration Technique
- Route: Subcutaneous injection only.
- Sites: Rotate between the abdomen (2 inches from navel), buttocks, outer upper arm, or thigh.
- Preparation: Reconstitute with 1 mL of 0.9% Sodium Chloride. The blue solution must be used immediately or within 2 hours of preparation.
Frequently Asked Questions (FAQs)
-
What is the exact FDA-approved indication for ZYCUBO?
ZYCUBO (copper histidinate) is indicated for the treatment of Menkes disease in pediatric patients. It is not indicated for the treatment of Occipital Horn Syndrome (OHS)
-
How significantly does ZYCUBO improve survival in infants?
In a pooled clinical analysis, pediatric patients who initiated ZYCUBO within the first four weeks of birth (Early Treatment cohort) demonstrated a 78% reduction in the risk of mortality compared to an untreated external control group. The median overall survival for this cohort was 177.1 months versus 17.6 months for the control group
-
What specific monitoring is required to prevent copper toxicity?
Because copper can accumulate in the body, clinicians must obtain baseline serum copper, ceruloplasmin, electrolytes, liver/kidney function, and a complete blood count (CBC). These levels should be monitored every 6 weeks for the first 6 months of therapy, and then every 3–6 months thereafter
-
How is ZYCUBO administered and stored?
ZYCUBO is for subcutaneous injection only. It is supplied as a lyophilized powder that must be reconstituted with 0.9% Sodium Chloride, USP. Once reconstituted, the solution must be used within 2 hours. Injection sites (abdomen, thighs, upper arms) should be rotated with each dose
-
Are there any known drug interactions or contraindications?
There are no contraindications listed in the FDA-approved label. Pharmacokinetic studies indicate that copper histidinate is not a substrate or inhibitor of major CYP450 enzymes. However, clinicians should monitor for potential interference with iron metabolism, which may lead to anemia
Regulatory and Investor Context
From an investment perspective, ZYCUBO represents a successful execution of a specialty orphan strategy.
- Designations: The FDA granted Breakthrough Therapy, Fast Track, and Priority Review status.
- Market Exclusivity: As an Orphan Drug, ZYCUBO receives 7 years of market exclusivity.
- Priority Review Voucher (PRV): Upon approval, the FDA issued a Rare Pediatric Disease PRV to Sentynl, which was subsequently transferred to Cyprium Therapeutics as part of the asset purchase agreement.
- Milestones: Cyprium stands to earn up to $129 million in tiered aggregate development and sales milestones from Sentynl.
Conclusion: A Future for Menkes Families
The approval of ZYCUBO (copper histidinate) is a watershed moment for the rare disease community. By achieving a 78% reduction in the risk of mortality and providing a decade-plus of additional life for many early-treated children, it transforms Menkes disease from a death sentence into a manageable chronic condition.
However, the “clinical success” of ZYCUBO is deeply dependent on early diagnosis. Rapid biochemical testing for low copper and ceruloplasmin levels, as well as the potential future inclusion of Menkes disease in Newborn Screening (RUSP) panels, will be essential to maximize the therapeutic benefit of this landmark treatment. For HCPs, the focus now shifts to vigilance: early suspicion, rapid initiation, and lifelong monitoring for copper-induced toxicity.
Disclaimer: For informational purposes only. Consult full prescribing information before prescribing or administering ZYCUBO.
Contact us for availability in India.
References
- U.S. Food and Drug Administration (FDA). “FDA Approves First Treatment for Children With Menkes Disease.” FDA News Release, January 12, 2026
- U.S. Food and Drug Administration (FDA). “ZYCUBO® (copper histidinate) for injection, for subcutaneous use.” Highlights of Prescribing Information, Revised January 2026. [NDA 211241].
- U.S. Food and Drug Administration (FDA). “NDA Approval Letter – Zycubo.” Reference ID: 531****, January 12, 2026
- Fortress Biotech, Inc. & Cyprium Therapeutics. “Fortress Biotech and Cyprium Therapeutics Announce U.S. FDA Approval of ZYCUBO® (copper histidinate), the First and Only Approved Treatment for Menkes Disease in the United States.” GlobeNewswire, January 13, 2026.
- Sentynl Therapeutics, Inc. “Sentynl Therapeutics Inc. Announces FDA Approval of ZYCUBO® (copper histidinate).” PR Newswire, January 13, 2026.
- Sentynl Therapeutics, Inc. “Sentynl Therapeutics Announces FDA Acceptance of CUTX-101 NDA Resubmission.” PR Newswire, December 15, 2025.
- Fortress Biotech, Inc. “Fortress Biotech and Cyprium Therapeutics Announce an Update on the NDA for CUTX-101 [Complete Response Letter].” GlobeNewswire, October 1, 2025.
- Fortress Biotech, Inc. “Fortress Biotech and Cyprium Therapeutics Announce U.S. FDA Acceptance and Priority Review of NDA for CUTX-101 for Treatment of Menkes Disease.” GlobeNewswire, January 6, 2025.
- Cyprium Therapeutics. “Cyprium Therapeutics… Completes Asset Transfer of CUTX-101 Copper Histidinate Product Candidate for Treatment of Menkes Disease, to Sentynl Therapeutics.” Biopharma Boardroom, December 7, 2023.
- Fortress Biotech, Inc. “Form 8-K: Report of Material Event [FDA Approval of Zycubo].” Securities and Exchange Commission Filing, January 13, 2026.
- Venkataraman, L., et al. “Intravenous AAV9-ATP7A plus subcutaneous copper histidinate optimizes outcomes in a lethal Menkes disease mouse model.” Science Advances, Vol. 11, No. 35, August 29, 2025.
- Kaler, S.G., et al. “Neurodevelopment and brain growth in classic Menkes disease is influenced by age and symptomatology at initiation of copper treatment.” Journal of Trace Elements in Medicine and Biology, Vol. 28, No. 4, 2014.
- Kaler, S.G., et al. “Neonatal Diagnosis and Treatment of Menkes Disease.” The New England Journal of Medicine, Vol. 358, 2008. (Cited in “Neurodevelopmental Outcomes…” ResearchGate).
- León-García, G., et al. “The T1048I mutation in ATP7A gene causes an unusual Menkes disease presentation.” BMC Pediatrics, Vol. 12, No. 150, 2012.
- Vonk, W.I.M., et al. “The copper-transporting capacity of ATP7A mutants associated with Menkes disease is ameliorated by COMMD1 as a result of improved protein expression.” Cellular and Molecular Life Sciences, Vol. 69, 2011.
- Dunleavy, Kevin. “FDA blesses Sentynl and Cyprium’s Zycubo as 1st treatment for Menkes disease.” Fierce Pharma, January 13, 2026.
- Manalac, Tristan. “Sentynl, Fortress Bounce Back With FDA Approval for Rare Pediatric Disease.” BioSpace, January 13, 2026.
- Halpern, Luke. “Copper Histidinate Becomes First, Only FDA-Approved Treatment for Menkes Disease.” Pharmacy Times, January 13, 2026.
- Ciccone, Isabella. “FDA Approves Subcutaneous Copper Histidinate as First Treatment for Pediatric Menkes Disease.” NeurologyLive, January 13, 2026.
- Taylor, Phil. “Sentynl gets first FDA approval for rare disease Menkes.” Pharmaphorum, January 13, 2026.
- Eckford, Catherine. “Sentynl secures US-first approval for rare Menkes disease.” European Pharmaceutical Review, January 13, 2026.
- Lewis, Ricki, PhD. “Menkes Disease Treatment Approved, After Three Decades of Testing.” DNA Science (PLOS Blogs), January 15, 2026.
- Patsnap Synapse. “A Historic First for Menkes Disease: How Zycubo’s FDA Approval is Redefining Rare Disease R&D.” January 21, 2026.
- U.S. Department of Health and Human Services (HHS). “Secretary Kennedy Adds Duchenne Muscular Dystrophy, Metachromatic Leukodystrophy to Newborn Screenings.” HHS Press Release, December 16, 2025.
- Indian Health Service. “HHS Takes a Lifesaving Step Forward for Newborns in Tribal Communities.” 2026 Announcements.
- Columbia University Irving Medical Center. “Gene Therapy May Offer New Hope for Infants with Rare, Fatal Disorder.” CUIMC News, October 30, 2025.
