In the current pharmaceutical landscape of 2026, the cost of entry is no longer just about brick and mortar—it is about the price of compliance. With the final implementation of Revised Schedule M by the CDSCO, the era of basic manufacturing is over. For entrepreneurs, PCD owners, and established firms, the CAPEX required to build a compliant facility from scratch has skyrocketed, often exceeding ₹10–15 Crores for even a mid-scale unit. Going with a Revised Schedule M compliant unit for rent in Haryana, near Gurugram.
Beyond the capital, the “Time-to-Market” is a silent killer. Setting up a new plant, obtaining environmental clearances, and securing drug licenses can take 18–24 months.
Renting a pre-certified, operational unit is the ultimate strategic shortcut. It allows you to bypass the construction phase and move directly into the production of high-quality, compliant formulations.
Unit Overview: Strategic Hub in Rewari, Haryana
Located in the heart of the Rewari industrial cluster, this pharmaceutical manufacturing unit offers a geographical and operational advantage that is hard to replicate. Haryana has emerged as a premier pharma destination due to its proximity to Delhi-NCR and its robust logistics corridor connecting to northern and western India.
- Location: Rewari, Haryana (Near NH-48)
- Total Land Area: 2,500 Sq. Yards
- Covered Area: 15,000 Sq. Ft. (Multi-level)
- Operational Status: Fully operational with active third-party contracts.
- Year of Commissioning: 2018 (Upgraded for Revised Schedule M in 2025).
The unit is positioned to serve the high-demand markets of Punjab, Rajasthan, and Delhi, making it an ideal distribution point for PCD and generic business models.
Regulatory & Compliance Readiness
The most critical asset of this facility is its Regulatory Shield. In a period where the CDSCO and State Drug Controllers are conducting rigorous risk-based inspections, this unit offers peace of mind.
Revised Schedule M Compliance
The facility has been upgraded to meet the 2026 standards, incorporating:
- Pharmaceutical Quality System (PQS): Documented risk management and product quality reviews.
- Environmental Control: Advanced HVAC systems with HEPA filtration ensuring ISO Class 7 and Class 8 air quality in core areas.
- Data Integrity: ALCOA+ compliant documentation systems with validated computerized storage for batch records.
Licensing Structure
- Valid Drug Licenses: Possession of Form 25 and Form 28.
- Loan License Support: The unit is optimized for “Loan License” arrangements. A new entity can apply for a loan license using this unit’s credentials, typically receiving approval within 15-30 days, significantly faster than a fresh manufacturing license.
Infrastructure & Technical Specifications
The plant is designed with a logical material and personnel flow to prevent cross-contamination—a core requirement of the new GMP norms.
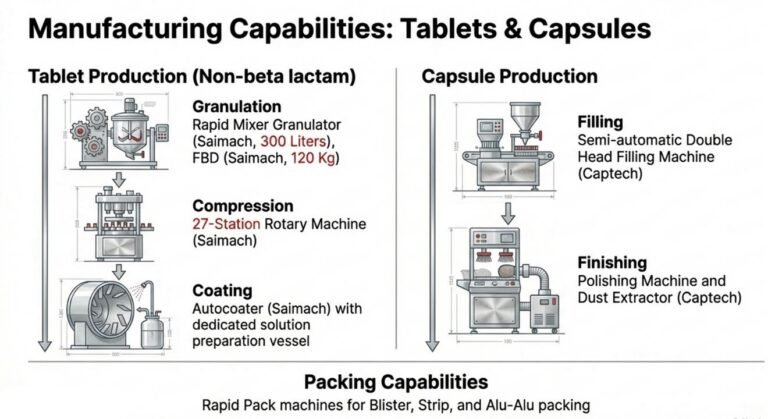
Dosage Forms & Installed Capacity
| Dosage Form | Installed Monthly Capacity | Key Machinery |
| Tablets | 15 Million Units | High-speed 27-station Double Rotary Compression, Auto-coater |
| Capsules | 8 Million Units | Automatic Capsule Filling (Size 0 & 2), De-dusting unit |
Technical Utility List
- Water System: Fully validated RO + EDI system with SS 316L distribution loop.
- HVAC: Dedicated AHUs for separate sections to ensure pressure differentials.
- Laboratory: Fully equipped QC lab with HPLC, UV-Spectrophotometer, and Stability Chambers.
Production Capacity & Scalability
While the current capacity is robust, the facility layout allows for modular expansion. There is dedicated space available for the installation of an additional Blister Packing line or a dedicated section for Nutra/Herbal production, should the renter wish to diversify.
The existing infrastructure is currently utilized at 60% capacity, offering immediate room for a new renter to scale up their own brands without further investment in utilities.
Existing Business Advantage: Third-Party Continuity
A unique feature of this unit is its active business ecosystem. Unlike an empty shell, this plant currently manufactures for 15+ established PCD and Third-Party clients.
- Risk Mitigation: The ongoing revenue from existing clients can offset a significant portion of the monthly lease and operational costs.
- Vendor Relationships: Established accounts with API and packaging material suppliers in the Rewari-Gurgaon belt ensure credit terms and priority supply.
Manpower & Operational Economics
Rewari offers a distinct cost advantage over hubs like Baddi or Ahmedabad.
- Skilled Workforce: Access to a pool of experienced Production Managers, QA/QC Chemists, and Machine Operators residing locally.
- Labor Costs: Competitive wage structures compared to NCR-Delhi.
- Utilities: Stable industrial power supply with a dedicated 125 KVA transformer and silent DG backup.
Why Rewari, Haryana Makes Commercial Sense
Haryana’s Pharmaceutical Policy provides a supportive environment for MSMEs. The strategic location in Rewari allows for:
- Logistics: Zero-mile access to the Delhi-Mumbai Industrial Corridor (DMIC).
- Regulatory Access: Proximity to the State Drug Controller office in Panchkula and CDSCO North Zone.
- Ecosystem: Immediate availability of corrugated box manufacturers, printers, and API distributors within a 20km radius.
Feature Summary Table
| Feature | Specification / Details |
| Compliance | Revised Schedule M (2026) / GMP Certified |
| Location | Rewari, Haryana |
| Area | 15,000 Sq. Ft. Covered |
| Power | 125 KVA (Dedicated) |
| Water | Purified Water System (SS 316L Loop) |
| Primary Sections | Tablets, and Capsules(Non-Beta) |
| QC Lab | Fully Instrumental & Microbiological |
| Entry Model | Lease / Rental with Loan License support |
Rent, Legal Structure & Entry Model
We offer a transparent entry model designed for serious pharmaceutical players.
- Monthly Rent: Competitive market rates (Contact for Quote based on area usage).
- Security Deposit: 6 Months.
- Legal Entity: The unit is owned by a Private Limited firm.
- Manufacturing Model: The renter can operate under a Loan License (Form 25A) or enter into a Management Contract to utilize the existing firm’s licenses for a faster start.
Frequently Asked Questions
-
Is the unit ready for the 2026 Revised Schedule M audits?
Yes. The HVAC, water systems, and documentation (PQS) have been upgraded and validated as of late 2025.
-
Can I bring my own machinery?
While the unit is fully equipped, there is “Plug & Play” provision for specialized machinery (e.g., Softgel or Injectable lines) in the expansion block.
-
How long does it take to start production?
If you opt for a Loan License, you can begin production within 6–8 weeks once the product approvals are transferred or granted.
-
Who is this unit ideal for?
It is perfect for PCD company owners looking to move into manufacturing, or exporters who need a compliant site for ROW (Rest of World) market audits.
Conclusion: Strategic Scalability
Building a pharma plant today is a 2-year gamble. Renting this Revised Schedule M compliant unit in Rewari is a strategic move that preserves your capital for marketing and R&D while ensuring your products meet the highest regulatory standards from Day 1. It is faster, safer, and economically superior to greenfield investments.
Take the next step in your pharma journey.
To schedule a confidential site visit or to review the Site Master File (SMF), contact our lead consultant.
Direct Contact: contact@laafon.com
Location: Rewari, Haryana.