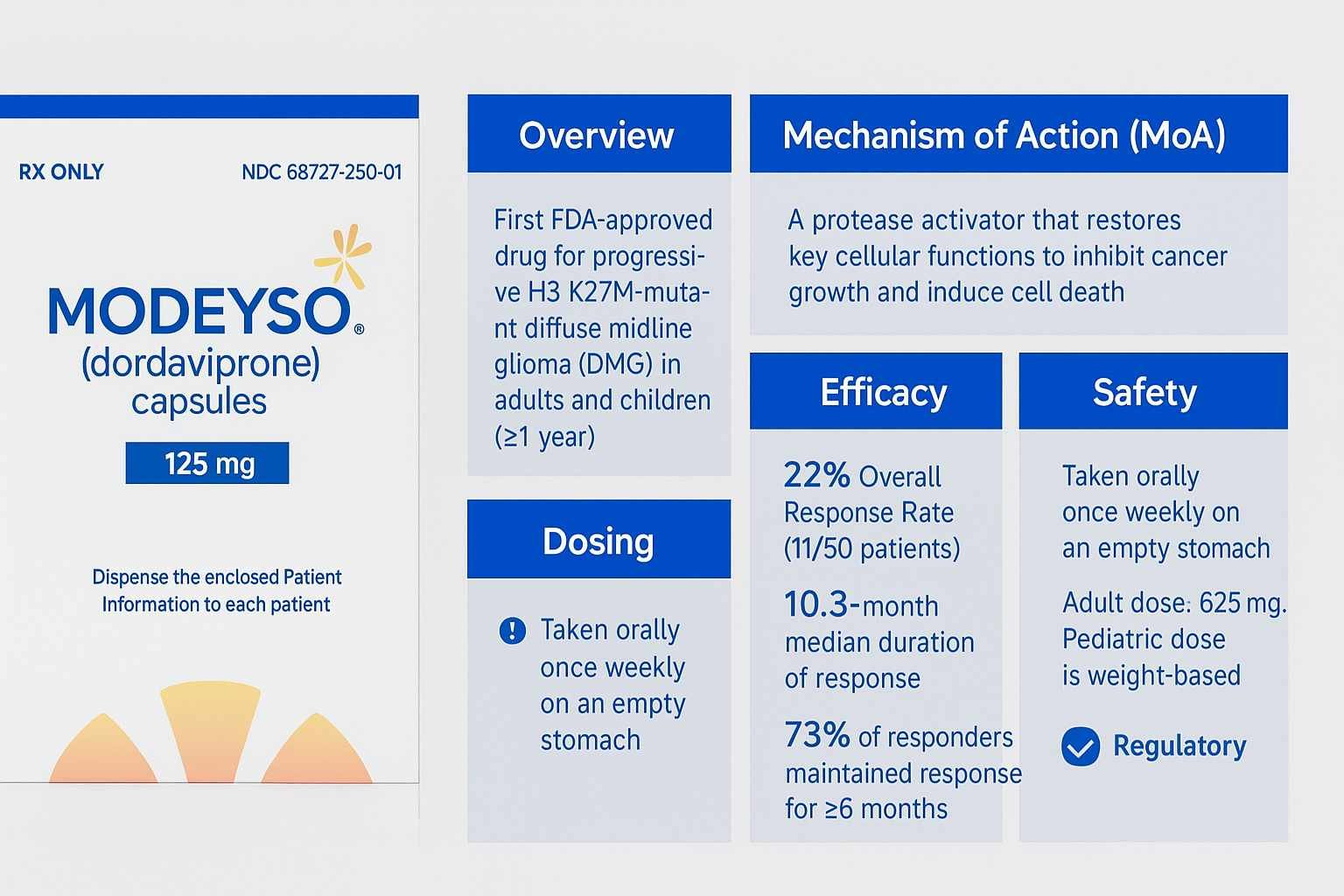
On August 6, 2025, the U.S. Food and Drug Administration (FDA) granted accelerated approval to MODEYSO (dordaviprone), a first-in-class protease activator for adult and pediatric patients aged one year and older with H3 K27M-mutant diffuse midline glioma (DMG) that has progressed following prior therapy [1]. This approval addresses a significant unmet need for this ultra-rare and aggressive central nervous system malignancy, which carries a poor prognosis. Dordaviprone is an oral, once-weekly capsule distributed by Jazz Pharmaceuticals [1]. The approval was based on an integrated analysis of 50 patients from five open-label studies, which demonstrated an overall response rate (ORR) of 22% (95% CI: 12, 36) and a median duration of response (DOR) of 10.3 months [1]. Key safety concerns include the potential for QTc interval prolongation, severe hypersensitivity reactions, and embryo-fetal toxicity [1]. Continued approval is contingent upon verification of clinical benefit in confirmatory trials assessing overall survival [1].
Overview of the Drug
MODEYSO (dordaviprone) is a novel, orally administered small molecule that functions as a protease activator of the mitochondrial caseinolytic protease P (ClpP) and an inhibitor of the dopamine D2 receptor [1]. Developed to target the specific biology of H3 K27M-mutant gliomas, its approval marks a significant advancement for a patient population with historically limited therapeutic options. The H3 K27M mutation is a hallmark of DMG, a high-grade glioma primarily affecting children and young adults, with a median survival of approximately one year from diagnosis. Dordaviprone is supplied as a 125 mg capsule for once-weekly administration [1].
Indications & Patient Selection
MODEYSO is indicated for the treatment of adult and pediatric patients 1 year of age and older with diffuse midline glioma harboring an H3 K27M mutation with progressive disease following prior therapy [1].
This indication received accelerated approval from the FDA based on surrogate endpoints of response rate and duration of response. Confirmatory trials are required to verify and describe the clinical benefit, particularly regarding overall survival [1].
Patient selection is critically dependent on the confirmed presence of an H3 K27M mutation in tumor specimens. The full prescribing information notes that an FDA-approved test for this mutation is not currently available, necessitating the use of laboratory-developed tests for diagnosis [1].
Dosing & Administration
MODEYSO should be administered orally once weekly on the same day each week. It must be taken on an empty stomach, defined as at least one hour before or three hours after food intake, as food can decrease its maximum concentration [1]. Treatment should continue until disease progression or unacceptable toxicity occurs [1].
- Adults: The recommended dosage is 625 mg (five 125 mg capsules) orally once weekly [1].
- Pediatrics (≥1 year of age): The dosage is based on body weight for patients weighing at least 10 kg. A recommended dosage has not been established for children weighing less than 10 kg [1].
- 10 kg to <12.5 kg: 125 mg once weekly
- 12.5 kg to <27.5 kg: 250 mg once weekly
- 27.5 kg to <42.5 kg: 375 mg once weekly
- 42.5 kg to <52.5 kg: 500 mg once weekly
- ≥52.5 kg: 625 mg once weekly
Capsules should be swallowed whole. For patients unable to do so, the capsules may be opened and the contents mixed with 15 to 30 mL of a liquid such as a sports drink, apple juice, lemonade, or water. The mixture should be administered within two hours of preparation [1].
Dose Adjustments:
- Adverse Reactions: Dose reductions are specified for QTc prolongation and other Grade 3 or 4 adverse reactions. For certain severe reactions or recurrent Grade 4 events, permanent discontinuation is required [1].
- Renal/Hepatic Impairment: While pharmacokinetic studies show increased dordaviprone exposure in patients with severe renal impairment or moderate hepatic impairment, the prescribing information does not provide specific dose adjustment recommendations for these populations [1].
- Missed Dose: If a dose is missed by two days or less, it should be taken as soon as possible. If missed by more than two days, the dose should be skipped, and the next dose taken at the regularly scheduled time [1].
- Vomiting: If vomiting occurs after a dose, an additional dose should not be taken. The next dose should be taken at the regularly scheduled time [1].
Mechanism of Action
Dordaviprone has a dual mechanism of action. It is a protease activator of the mitochondrial caseinolytic protease P (ClpP) and an inhibitor of the dopamine D2 receptor [1]. The H3 K27M mutation in diffuse midline gliomas leads to a global loss of histone H3 lysine 27 (H3 K27) trimethylation, a critical epigenetic mark for gene regulation [1]. In vitro studies in H3 K27M-mutant glioma models have shown that dordaviprone activates the integrated stress response, induces apoptosis, and alters mitochondrial metabolism. These actions ultimately lead to the restoration of histone H3 K27 trimethylation, counteracting the primary oncogenic effect of the mutation and exhibiting antitumor activity [1].
Clinical Evidence
The efficacy of MODEYSO was established based on an integrated analysis of 50 patients from five multicenter, open-label, non-randomized clinical studies (ONC006, ONC013, ONC014, ONC016, and ONC018) [1]. Eligible patients had H3 K27M-mutant DMG with progressive, measurable disease following prior therapy, were at least 90 days post-radiation, and had a Karnofsky/Lansky Performance Status (KPS/LPS) score of ≥60 [1].
The efficacy population had a median age of 31 years (range: 9 to 70), with 6% being pediatric patients (<17 years). The majority of patients (72%) were treated at their first recurrence, and primary tumors were located in the thalamus (52%) or other non-thalamic midline regions (48%) [1].
The major efficacy outcome was Overall Response Rate (ORR), as assessed by a blinded independent central review (BICR) using Response Assessment in Neuro-Oncology (RANO 2.0) criteria. The median time to response was 3.6 months [1].
Of course! Here is the table you requested.
Efficacy Results (BICR, RANO 2.0)
| Efficacy Parameter | Result (N=50) | 95% Confidence Interval |
| Overall Response Rate (ORR) | 22% | (12, 36) |
| Partial Response (PR) | 16% | N/A |
| Minor Response (MR) | 6% | N/A |
| Median Duration of Response (DOR) | 10.3 months | (7.3, 15.2) |
| Patients with DOR ≥6 months | 73% | N/A |
| Patients with DOR ≥12 months | 27% | N/A |
Safety Profile
The safety of MODEYSO was evaluated in a pooled population of 376 adult and pediatric patients with glioma from four open-label studies. The median duration of exposure was not specified, but 35% of patients were exposed for at least 6 months and 17% for at least one year [1].
- Most Common Adverse Reactions (≥20%): The most frequently reported adverse reactions were fatigue (34%), headache (32%), vomiting (24%), nausea (24%), and musculoskeletal pain (20%) [1].
- Serious Adverse Reactions: Serious adverse reactions occurred in 33% of patients. The most common (occurring in >2% of patients) were hydrocephalus (5%), vomiting (4.3%), headache (3.2%), seizure (2.4%), and muscular weakness (2.1%) [1].
- Fatal Adverse Reactions: Fatal adverse events occurred in 1% of patients and included cardiac arrest, intracranial hemorrhage, and encephalopathy [1].
- Discontinuation: Permanent discontinuation due to an adverse reaction occurred in 2.1% of patients, most frequently due to a confusional state [1].
Warnings and Precautions:
- Hypersensitivity: MODEYSO can cause severe hypersensitivity reactions, including rash, hives, fever, hypotension, wheezing, and angioedema. Grade 3 hypersensitivity occurred in 0.3% of patients. MODEYSO should be interrupted if hypersensitivity is suspected and permanently discontinued for serious reactions [1].
- QTc Interval Prolongation: Dordaviprone causes concentration-dependent QTc interval prolongation, which increases the risk of torsades de pointes and sudden death. In the safety population, 6% of patients with a post-baseline ECG had a QTc increase of >60 msec. ECGs and serum electrolytes must be monitored before initiating treatment and periodically thereafter. Concomitant use with other QT-prolonging drugs should be avoided [1].
- Embryo-fetal Toxicity: Based on animal studies, MODEYSO can cause fetal harm. Embryo-fetal mortality and structural abnormalities were observed in rats and rabbits. Females of reproductive potential must be advised of the risk and should use effective contraception during treatment and for one month after the final dose. Male patients with female partners of reproductive potential should also use effective contraception during the same period [1].
Boxed Warning / REMS: MODEYSO does not have a boxed warning or a Risk Evaluation and Mitigation Strategy (REMS) program [1].
Special Populations
- Pregnancy: MODEYSO can cause fetal harm and should not be used during pregnancy unless the potential benefit justifies the potential risk to the fetus. Pregnancy status should be verified before initiation of therapy [1].
- Lactation: There are no data on the presence of dordaviprone in human milk. Due to the potential for serious adverse reactions in a breastfed child, women should not breastfeed during treatment and for one week after the last dose [1].
- Pediatric Use: Safety and effectiveness have been established in children aged one year and older. The safety profile in 154 pediatric patients (aged 3 to 17) was consistent with the overall population, with no new safety signals observed [1].
- Geriatric Use: Clinical studies did not include enough patients aged 65 and over to determine if they respond differently from younger patients [1].
- Fertility: The prescribing information notes that MODEYSO may cause fertility problems in both males and females based on its mechanism of action [1].
Drug–Drug Interactions & Contraindications
Contraindications: There are no contraindications for MODEYSO [1].
Drug Interactions:
- CYP3A4 Inhibitors (Strong and Moderate): These drugs increase dordaviprone exposure, which may increase the risk of adverse reactions. Concomitant use should be avoided. If unavoidable for patients weighing ≥52.5 kg, the MODEYSO dose should be reduced: to 375 mg weekly with a strong inhibitor, and to 500 mg weekly with a moderate inhibitor [1].
- CYP3A4 Inducers (Strong and Moderate): These drugs decrease dordaviprone exposure, which may reduce its antitumor activity. Concomitant use should be avoided [1].
- QTc-Prolonging Drugs: Concomitant use may increase the risk of QTc-associated arrhythmias and should be avoided. If unavoidable, administration should be separated in time, and ECG monitoring should be increased [1].
Pharmacokinetics & Pharmacodynamics
- Absorption: Following oral administration, the median time to maximum plasma concentration (Tmax) is 1.4 hours. A high-fat meal decreases Cmax by 40% with no effect on total exposure (AUC) [1].
- Distribution: Dordaviprone has an apparent volume of distribution of 450 L and is highly protein-bound (95% to 97%) in plasma [1].
- Metabolism: It is primarily metabolized by CYP3A4 [1].
- Excretion: The mean terminal half-life is 11 hours. Approximately 70% of a dose is recovered in urine and 20% in feces, with no notable unchanged drug in either [1].
- Pharmacodynamics: Dordaviprone causes a concentration-dependent prolongation of the QTcF interval. At 1.2 times the maximum recommended dose, the estimated mean change was 11.8 msec [1].
Regulatory Path & Milestones
MODEYSO was approved under the FDA’s Accelerated Approval pathway, which allows for earlier approval of drugs that treat serious conditions and fill an unmet medical need based on a surrogate endpoint. The approval was based on ORR and DOR [1].
As part of this pathway, the manufacturer, Jazz Pharmaceuticals, is required to conduct postmarketing studies to verify the clinical benefit. This includes a multiregional, randomized clinical trial with overall survival (OS) as the primary endpoint.
Chimerix, Inc., the original developer, was granted a Rare Pediatric Disease Priority Review Voucher upon approval, a program intended to encourage the development of new drugs for rare pediatric diseases.
Global Status
- European Medicines Agency (EMA): UNKNOWN. The provided documents do not contain information on the regulatory status of dordaviprone in the European Union.
- Central Drugs Standard Control Organisation (CDSCO), India: UNKNOWN. The provided documents do not contain information on the regulatory status of dordaviprone in India.
Practical Considerations
Clinicians prescribing MODEYSO must ensure that the H3 K27M mutation status of the tumor has been confirmed [1]. Pre-treatment screening should include an ECG and measurement of serum electrolytes, with periodic monitoring throughout treatment [1]. Patients must be counseled on the importance of taking the medication on an empty stomach and on the risks of QTc prolongation, hypersensitivity, and embryo-fetal toxicity. For patients who cannot swallow capsules, clear instructions for mixing the contents with liquid are provided in the patient information leaflet [1].
Limitations & Evidence Gaps
The primary limitation of the current evidence is that the approval is based on a single-arm, integrated analysis of a relatively small number of patients (N=50) using surrogate endpoints (ORR and DOR) rather than overall survival [1]. The open-label, non-randomized design introduces potential biases. The efficacy and safety in children under one year of age or weighing less than 10 kg are unknown. Furthermore, specific dose adjustments for patients with renal or hepatic impairment have not been established. The results of the mandatory confirmatory trial are needed to definitively establish the clinical benefit of dordaviprone in this patient population.
Patient-Friendly Summary
What is MODEYSO (dordaviprone)?
MODEYSO is a new prescription medicine for adults and children (1 year and older) with a specific type of brain tumor called diffuse midline glioma, or DMG. It is used when the tumor has a particular genetic marker (an “H3 K27M mutation”) and has grown after previous treatments. Because this is a rare and serious cancer with few options, MODEYSO was approved through a special FDA pathway called “accelerated approval.”
How does it work and how is it taken?
MODEYSO works in a new way to target the cancer cells, aiming to cut off their energy supply and correct the genetic problem that helps them grow. It is a capsule taken by mouth once a week on an empty stomach. If you have trouble swallowing pills, the capsule can be opened and mixed with certain liquids like apple juice or water.
What did the studies show?
In a key study of 50 patients, tumors shrank in about 1 out of 5 people (22%) who took MODEYSO. For those who responded, the effect lasted for a median of about 10 months.
What are the most important side effects?
The most common side effects are feeling tired, headache, vomiting, nausea, and muscle or joint pain. MODEYSO can also cause more serious problems, including changes in your heart’s electrical rhythm (called QTc prolongation) and serious allergic reactions. Because it may harm an unborn baby, you must use effective birth control while taking it. Your doctor will monitor your heart and overall health closely during treatment. Always discuss any side effects or concerns with your healthcare team.
FAQs
What is MODEYSO (dordaviprone)?
MODEYSO (dordaviprone) is a first-in-class, oral, once-weekly medication that received FDA accelerated approval to treat adults and children (≥1 year) with H3 K27M-mutant diffuse midline glioma that has progressed after prior therapy.
What is the mechanism of action of dordaviprone?
Dordaviprone is a protease activator of the mitochondrial protein ClpP and an inhibitor of the dopamine D2 receptor. This dual action is believed to restore histone H3 K27 trimethylation and induce apoptosis in H3 K27M-mutant glioma cells.
What is the efficacy of MODEYSO in diffuse midline glioma?
In an integrated analysis of 50 patients, MODEYSO demonstrated an overall response rate (ORR) of 22% and a median duration of response (DOR) of 10.3 months, based on a blinded independent central review.
What are the key warnings for MODEYSO?
The main warnings and precautions for MODEYSO include the risk of QTc interval prolongation, severe hypersensitivity reactions, and embryo-fetal toxicity. ECG and electrolyte monitoring are required.
How is MODEYSO dosed?
For adults, the dose is 625 mg orally once weekly. For children weighing 10 kg or more, the dose is weight-based. It must be taken on an empty stomach.
Disclaimer:
Disclaimer: All the information and articles available on this site are for educational purposes only. The information given here should not be used for the diagnosis or treatment of any health problem or disease without expert advice. The advice of a qualified medical practitioner should always be sought for medical examination and treatment.
Medically Reviewed by:

Dr. Yogesh Chaudhary (B. Pharma)
Senior Pharmacist at S.N. Medical College, Agra-(UP)
References:
- [1] MODEYSO™ (dordaviprone) capsules, for oral use. Full Prescribing Information. Jazz Pharmaceuticals, Inc. August 2025.
- Chi, A. S., Tarapore, R. S., Hall, M. D., Shonka, N., Gardner, S., Umemura, Y., Sumrall, A., Khatib, Z., Mueller, W., Kline, C., Zaky, W., Margol, A., & Johnston, J. (2022). Phase II study of ONC201 in pediatric H3 K27M-mutant glioma. Neuro-Oncology, *24*(Supplement_1), i13-i14. [
https://doi.org/10.1093/neuonc/noac079.046] - ClinicalTrials.gov. (2023). *A Study of ONC201 in H3 K27M-Mutant Glioma (ACTION)*. National Library of Medicine. Retrieved August 23, 2025, from [
https://clinicaltrials.gov/study/NCT05476939] - Arrillaga-Romany, I., Odia, Y., Prabhu, V. V., Tarapore, R. S., Merdinger, K., Stogniew, M., Oster, W., Allen, J. E., & Wen, P. Y. (2020). A phase 2 study of the first imipridone ONC201 in recurrence of glioblastoma and other gliomas. Journal of Clinical Oncology, *38*(15_suppl), 2505. [
https://doi.org/10.1200/JCO.2020.38.15_suppl.2505] - 4. Louis, D. N., Perry, A., Wesseling, P., Brat, D. J., Cree, I. A., Figarella-Branger, D., Hawkins, C., Ng, H. K., Pfister, S. M., Reifenberger, G., Soffietti, R., von Deimling, A., & Ellison, D. W. (2021). The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro-Oncology, *23*(8), 1231–1251.[
https://doi.org/10.1093/neuonc/noab106] - Mackay, A., Burford, A., Carvalho, D., Izquierdo, E., Fazal-Salom, J., Taylor, K. R., Bjerke, L., Clarke, M., Vinci, M., Nandhabalan, M., Temelso, S., Popov, S., Molinari, V., Raman, P., Waanders, A. J., Han, H. J., Gupta, S., Marshall, L., Zacharoulis, S., … Jones, C. (2017). Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell, *32*(4), 520–537.e5. [
https://doi.org/10.1016/j.ccell.2017.08.017] - 6. ClinicalTrials.gov. (2024). A Study of ONC206 in Recurrent and Rare Primary Central Nervous System Neoplasms. National Library of Medicine. Retrieved August 23, 2025, from [
https://clinicaltrials.gov/study/NCT04541082] - Kline, C. L. B., Van den Heuvel, A. P. J., Allen, J. E., Prabhu, V. V., Dicker, D. T., & El-Deiry, W. S. (2016). ONC206, an Imipridone Derivative, Induces Cell Death through Activation of the Integrated Stress Response in Serous Ovarian Cancer Cell Lines. Journal of Clinical Oncology, *34*(15_suppl), e17085. [
https://ascopubs.org/doi/10.1200/JCO.2016.34.15_suppl.e17085] - 8. Prabhu, V. V., Morrow, S., Rahman Kawakibi, A., Zhou, L., Ralff, M. D., Ray, J., Jhaveri, A., Ferrarini, I., Lee, Y., Parker, C., Lopes, E. C., Tardy, C. M., Wagner, J., Zloza, A., Khushalani, N. I., Savage, J. E., Oster, W., Allen, J. E., & El-Deiry, W. S. (2020). ONC201 and imipridones: Anti-cancer compounds with clinical efficacy. Neoplasia, *22*(12), 725–744. [
https://www.sciencedirect.com/science/article/pii/S1476558620301512?via%3Dihub] - Graves, P. R., Aponte-Collazo, L. J., Fennell, E. M. J., Graves, A. C., Hale, A. E., Dicheva, N., Herring, L. E., Gilbert, T. S. K., East, M. P., McDonald, I. M., Lockett, M. R., Ashamalla, H., Moorman, N. J., Ippolito, J. E., & Allen, J. E. (2019). Mitochondrial Protease ClpP is a Target for the Anticancer Compounds ONC201 and Related Analogues. ACS Chemical Biology, *14*(5), 1020–1029. [
https://pubs.acs.org/doi/10.1021/acschembio.9b00222]