Introduction:
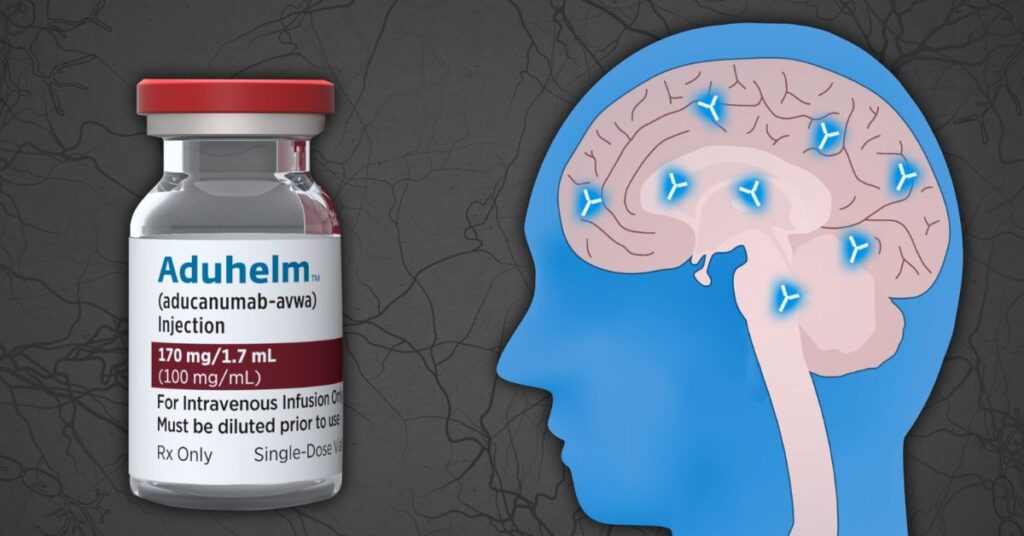
Aduhelm aducanumab drug is a medication in an injectable form that contains Aducanumab 170mg /1.7 ml (100mg/ml). It is used to treat Alzheimer’s disease. Aducanumab is a monoclonal antibody. It targets the amyloid protein that clumps into plaques in the brain of Alzheimer’s patients. It is considered the main cause of disease.
The US FDA granted accelerated approval for Aduhelm (Aducanumab-avwa) on June 7, 2021, as the first and only treatment for Alzheimer’s disease. It is to address a defining pathology of Alzheimer’s disease by minimizing amyloid beta plaques in the brain.
Understanding Alzheimer’s Disease
Alzheimer’s disease is a neurodegenerative disease that causes dementia and affects millions of people worldwide. This is the most common reason for dementia in people. The prevalence of this disease differs by several factors like region, age, and other risk factors.
In the United States, according to a study more than 6 million people of all ages are suffering from Alzheimer’s disease. The number of affected patients is doubling every 5 years after the age of 65.
Alzheimer’s disease is a trigger for high mortality and morbidity rates due to its impact on the memory of a patient. It is also responsible for economic losses. It has a very bad impact on society.
Mechanism of action of Aduhelm (aducanumab)
Aduhelm is a trade name for the drug aducanumab which is a monoclonal antibody. It targets amyloid beta(Aβ) present in the brain. Amyloid beta is a protein that is considered to be responsible for the development of Alzheimer’s disease. It works by binding to Amyloid beta plaques and eases the clumps in the brain.
Amyloid plaques are clumps of Aβ protein that are found in the brain of Alzheimer’s disease patients. These plaques play the role of damaging nerve cells, and they contribute to cognitive decay associated with the disease. Aduhelm helps to simply plaques from the brain that help to slow Alzheimer’s disease growth.
Clinical trials and research supporting Aduhelm’s (aducanumab) efficacy
There were some clinical trials were conducted on the drug aducanumab that are listed below with descriptions:
1. A clinical trial with the title ‘The EMERGE‘ was conducted in 2016 by Sevigny et al. It was a clinical trial study to investigate the efficacy of aducanumab as an anti-amyloid therapy for Alzheimer’s disease. The study shows the following outcomes:
- This Phase 3 clinical trial evaluated the efficacy and safety of Aducanumab in individuals with early Alzheimer’s disease.
- Aducanumab may help restore neurological function in patients with Alzheimer’s disease by minimizing amyloid plaques and restoring neuronal calcium permeability.
- Patients treated with Aducanumab showed a slower cognitive decline compared to the placebo group.
2. Another clinical trial ‘ENGAGE’ on Aducanumab was carried out in 2019 by Mintun et al. It was to know the efficacy of the aducanumab drug. It was a Phase III study with the objective to evaluate the efficacy of the experimental drug aducanumab(BIIB37). The outcomes found from the study are listed here;
- The study involved individuals with early Alzheimer’s disease and administered different doses of Aducanumab.
- Findings showed a reduction in amyloid beta plaques in the brains of patients receiving higher doses of Aducanumab, compared to the placebo group.
3. A Clinical trial namely “Biogen’s Clinical Development Program for Aducanumab: Engagement, Efficacy, and Safety Results” was carried out by Cummings in 2019.
This study was conducted by Biogen and Eisai companies. The outcomes of this study were:
- This paper provides an overview of the clinical development program conducted by Biogen for Aducanumab.
- It discusses data from multiple studies, including Phase 1b, Phase 2, and Phase 3 trials.
- The results demonstrate Aducanumab’s ability to reduce amyloid beta plaques and suggest a potential impact on slowing cognitive decline.
The Controversy Surrounding Aduhelm
There have been criticisms of the approval process for Aduhelm (Aducanumab). According to the New York Times article “A report by the US House committee challenges the FDA’s approval of Alzheimer’s drug Aduhelm and faults the FDA for its approval process”. The report said that the FDA’s approval process for Aduhelm was “rife with irregularities” and criticized Biogen for setting an “unjustifiably high price”. The criticism surrounding Aduhelm’s approval may have been avoided had the FDA adhered to its own guidance and internal practices
Some people also complain about the efficacy of Aduhelm’s effectiveness in Alzheimer’s disease patients. They give arguments that it is not justified at this price which is more than $15000 for one-year treatment.
Aduhelm’s (aducanumab) Potential Benefits
According to FDA Aduhelm is the first and only drug to treat Alzheimer’s disease in the United States. Therefore, the drug is no less than a miracle for patients. It has several benefits that can be concluded as;
- Slowing cognitive decline in Alzheimer’s patients
- Enhancing Brain Function and Memory
- Improving the quality of life for patients and caregivers
Common side effects of Aduhelm (aducanumab)
Some common side effects of Aduhelm (aducanumab) are as below:
- Headache disorder
- Amyloid-related imaging abnormality
- Acute confusion
- Dizziness
- Visual changes
- Nausea
- Falls
- Amyloid-related imaging abnormality with edema
- Amyloid-related imaging abnormality with microhemorrhage and superficial siderosis
Patient Experiences with Aduhelm (aducanumab)
As per some articles in different magazines on different Alzheimer’s disease patients who have been on the course of Aduhelm drug, a few of them are below;
Geri Taylor: Geri Taylor was diagnosed with mild cognitive impairment in 2012. She participated in a clinical trial for Aduhelm and was on the drug for three years. During that time, she declined very slowly. She was able to maintain normal daily activities and live and take care of herself. After she stopped taking the drug, she started to decline more quickly
John Archambault: John Archambault was the first patient to be treated with Aduhelm outside of clinical trials. He was diagnosed with Alzheimer’s disease in 2019. He said that he felt “very lucky and very happy” to be able to take the drug. He believes that it has helped him to slow the progression of his disease.
Mary Smith: Mary Smith was diagnosed with Alzheimer’s disease in 2018. She has been taking Aduhelm for six months. She said that she has noticed a difference in her cognitive function since starting the Aduhelm drug. She is able to remember things better and she is less forgetful.
Frequently Asked Questions (FAQs)
What is Aduhelm and how does it work?
Aduhelm (aducanumab) is a monoclonal antibody medicine that was approved by the U.S. Food and Drug Administration (FDA) on the 7th of June 2021, it was approved for the treatment of Alzheimer’s disease. It targets the Amyloid beta protein in the brain that is responsible for Alzheimer’s disease.
Who is eligible for Aduhelm treatment?
In the United States, Aduhelm is only covered by Medicare Part B if the patient is enrolled in a clinical trial approved by the Centers for Medicare & Medicaid Services (CMS) or supported by the National Institutes of Health (NIH).
What are the potential side effects of Aduhelm?
Aduhelm has some common side effects including headache, fatigue, dizziness, brain swelling, etc.
How much does Aduhelm cost and is it covered by insurance?
According to the National Council on Aging, Aduhelm costs $28,200 per year. Medicare Part B will cover 80% of the drug cost, and Medicare Supplement plans will cover the remaining 20% of coinsurance.
Conclusion
Aduhelm (aducanumab) is the first drug approved by the FDA in June 2021 for the treatment of Alzheimer’s disease. Since it was the first and only medicine for this disease. However, there are some controversies associated with the Aduhelm drug such as its cost is one of the main points and its effectiveness on Alzheimer’s disease patients.
It is a breakthrough for Alzheimer’s disease patients, and studies are continuing on the drug’s efficacy. it is hope for patients with Alzheimer’s disease.
References:
- FDA’s Decision to Approve New Treatment for Alzheimer’s Disease [FDA]
- Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention by Chengxuan Qiu, MD, PhD [PMC (nih.gov)]
- Dementia-Key Facts [WHO]
- Aducanumab, a Novel Anti-Amyloid Monoclonal Antibody, for the Treatment of Alzheimer’s Disease: A Comprehensive Review [ PMC (nih.gov)]
- Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease[SpringerLink]
- Biogen/Eisai Halt Phase 3 Aducanumab Trials [ALZFORUM]
- Our Experience With Aduhelm: Inside the Clinical Trial [being-patient]