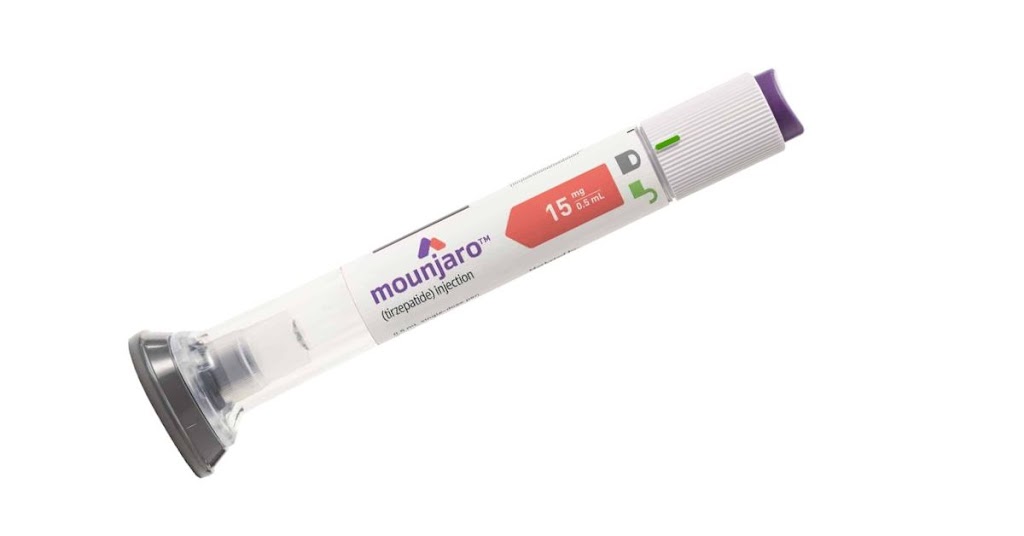
Unveiling Mounjaro (Tirzepatide): A Potential Breakthrough in Diabetes Treatment
Mounjaro (Tirzepatide): A diabetes and weight loss drug Introduction: Diabetes, a chronic metabolic disorder affecting millions of people worldwide, poses severe health challenges and demands effective treatment as soon as possible. The global prevalence of diabetes has been steadily rising highlighting the urgent need for innovative therapeutic interventions. Fortunately, medical advancements have introduced a promising […]
Unveiling Mounjaro (Tirzepatide): A Potential Breakthrough in Diabetes Treatment Read More »